Acemannan
Acemannan is a D-isomer mucopolysaccharide in aloe vera leaves. This compound has potential immunostimulant,[1] antiviral, antineoplastic, and gastrointestinal properties.[2]
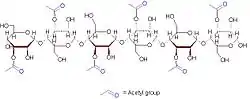 A representative 6-sugar fragment of acemannan | |
| Names | |
|---|---|
| IUPAC name
(2S,3S,4R,5S,6S)-6-[(2R,3R,4R,5S,6R)-6-[(2R,3S,4R,5S,6R)-5-acetamido-6-[(2R,3R,4R,5S,6R)-4-acetyloxy-6-[(2R,3R,4R,5S,6R)-4-acetyloxy-6-[(2R,3R,4R,5S,6S)-4-acetyloxy-5-hydroxy-2-(hydroxymethyl)-6-methoxyoxan-3-yl]oxy-5-hydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-5-hydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-4-hydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-4-acetyloxy-5-hydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-4-acetyloxy-3-[(2R,3S,4R,5R,6R)-4-acetyloxy-5-[(2R,3S,4R,5R,6R)-4-acetyloxy-3-hydroxy-6-(hydroxymethyl)-5-methoxyoxan-2-yl]oxy-3-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-5-hydroxyoxane-2-carboxylate | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.122.396 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C66H100NO49 | |
| Molar mass | 1691.484 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Chemical structure and properties
Acemannan's monomer is mannoacetate linked by β-1,4-glycosidic bonds.[3][4] This polymer is hydrophilic.
Immunostimulant properties
Acemannan has been demonstrated to induce macrophages to secrete interferon (IFN), tumor necrosis factor-α (TNF-α) and interleukins (IL-1); therefore, it might help to prevent or abrogate viral infection. These three cytokines are known to cause inflammation, and interferon is released in response to viral infections. In vitro studies have shown acemannan to inhibit HIV replication; however, in vivo studies have been inconclusive.
Acemannan is currently being used for treatment and clinical management of fibrosarcoma in dogs and cats. Administration of acemannan has been shown to increase tumor necrosis and prolonged host survival; the animals have demonstrated lymphoid infiltration and encapsulation.[5]
The compound has been found to have an LD50 of >80 mg/kg and LC50 >5,000 mg/kg IV.[6]
References
- Ebadi, Manuchair (2006-09-06). Pharmacodynamic Basis of Herbal Medicine (Second ed.). ISBN 978-1-4200-0645-2. Retrieved 23 May 2015.
- Pubchem. "SID 596005 – PubChem". nih.gov. Retrieved 23 May 2015.
- "Archived copy". Archived from the original on 2009-09-27. Retrieved 2009-04-02.
{{cite web}}: CS1 maint: archived copy as title (link) - "Acemannan Immunostimulant". drugs.com. Retrieved 23 May 2015.
- Harris, C; Pierce, K; King, G; Yates, K. M.; Hall, J; Tizard, I (1991). "Efficacy of acemannan in treatment of canine and feline spontaneous neoplasms". Molecular Biotherapy. 3 (4): 207–13. PMID 1768373.
- "MSDS: Acemannan Immunostimulant". Archived from the original on 2008-07-03. Retrieved 2009-04-02.