Acetyl cyanide
Acetyl cyanide is the organic compound with the formula CH3C(O)CN. It is an acyl cyanide. Acetyl cyanide is a colorless liquid.[2]
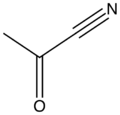 | |
| Names | |
|---|---|
| Preferred IUPAC name
Acetyl cyanide[1] | |
| Systematic IUPAC name
Ethanoyl cyanide | |
| Other names
2-Oxopropanenitrile[1] Pyruvonitrile Propanenitrile, 2-oxo- α-Oxopropionitrile Oxopropionitrile Oxypropionitrile Pyruvic acid nitrile 2-Oxopropionitrile 2-Oxopropiononitrile | |
| Identifiers | |
3D model (JSmol) |
|
| 1737633 | |
| ChemSpider | |
| ECHA InfoCard | 100.010.146 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H3NO | |
| Molar mass | 69.063 g·mol−1 |
| Appearance | Clear, yellow liquid |
| Density | 0.9745 g/cm3 |
| Boiling point | 92.3 °C (198.1 °F; 365.4 K) |
| Vapor pressure | 51.9300003051758 mmHg |
Refractive index (nD) |
1.3764 |
| 40.86 Å2 | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Ingestion hazards |
Toxic if swallowed |
Inhalation hazards |
Toxic if inhaled. Causes respiratory tract irritation |
Eye hazards |
Causes eye irritation |
Skin hazards |
May be harmful if absorbed through skin. Causes skin irritation. |
| GHS labelling: | |
  | |
| Danger | |
| H225, H301, H315, H331, H335, H401, H412 | |
| P210, P261, P273, P301+P310, P311 | |
| NFPA 704 (fire diamond) | |
| Flash point | 14.44 °C (57.99 °F; 287.59 K) |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Structure
Its structure was determined through the use of electron diffraction intensities and rotational spectroscopy.[3]
Reactions
Two main types of reactions can occur with acetyl cyanide as a reactant; aldol condensation and enolate substitution. Aldol condensation can occur when acetyl cyanide reacts with (Z)-but-2-enal to form (2E,4E)-hexa-2,4-dienoyl cyanide:
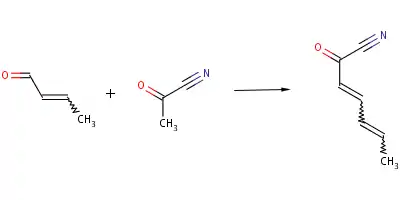
The photochemical and thermal reactions of acetyl cyanide have been extensively studied. For example, formyl cyanide does not undergo unimolecular decomposition to HCN and CO spontaneously. However, acetyl cyanide, also a member of this family, breaks down through this unimolecular decomposition at 470 °C. This reaction occurs through decarbonylation. This division of the molecule to a ketone and hydrogen cyanide were noted to be under competitive circumstances. This caused a study of the thermal unimolecular reactions that acetyl cyanide undergoes.
The unimolecular decompositions that acetyl cyanide undergo have been confirmed to be less energetically favorable than the molecule undergoing isomerization to acetyl isocyanide. However, through other photolysis experiments have resulted in the formation of a CN radical through acetyl cyanide decomposing into CH3CO + CN or CH3COCN.[4]
Synthesis
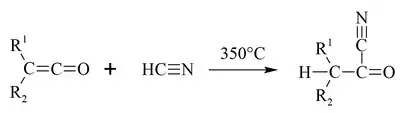
Acetyl cyanide is prepared from acetyl chloride and cyanide sources, often in the presence of copper catalysts.[2] Acetyl cyanide is also synthesized at 350 °C from ketene and hydrogen cyanide.[4]
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 796–797, 903. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Morris, Joel (2001). "Acetyl Cyanide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.ra026. ISBN 0471936235.
- Sugié, Masaaki; Kuchitsu, Kozo (1974). "Molecular structure of acetyl cyanide as studied by gas electron diffraction". Journal of Molecular Structure. 20 (3): 437–448. Bibcode:1974JMoSt..20..437S. doi:10.1016/0022-2860(74)85121-5.
- R. Sumathi; Minh Tho Nguyen (1998). "Theoretical Study on Unimolecular Reactions of Acetyl Cyanide and Acetyl Isocyanide" (PDF). J. Phys. Chem. A. 102: 412–421. Archived from the original (PDF) on 17 April 2007. Retrieved 7 March 2022.
Further reading
- Lide, David R., W. M. Haynes, and Thomas J. Bruno, eds. CRC Handbook of Chemistry and Physics. 93rd ed. Boca Raton, FL: CRC, 2012. Web. 17 October 2012.
- "1737633 | C3H3NO". Chemspider.com. Retrieved 7 March 2022.
