Acetyl nitrate
Acetyl nitrate is the organic compound with the formula CH3C(O)ONO2. It is classified as the mixed anhydride of nitric and acetic acids. It is a colorless explosive liquid that fumes in moist air.
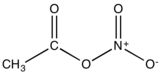 | |
| Names | |
|---|---|
| Preferred IUPAC name
Acetic nitric anhydride | |
| Other names
Acetyl nitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H3NO4 | |
| Molar mass | 105.05 |
| Appearance | colorless liquid |
| Density | 1.24 g/cm3 (15 °C) |
| Boiling point | 22 °C at 70 Torr [1] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
explosion |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis and reactions
It is prepared from acetic anhydride and dinitrogen pentoxide or with nitric acid:
- (CH3CO)2O + HNO3 → CH3C(O)ONO2 + CH3COOH
It hydrolyzes in moist air to acetic acid and nitric acid. Alternatively, nitric acid adds to ketene.
It is used for some nitrations and nitrolysis reactions.[2] It acetylates amines, akin to the behavior of acetyl chloride:
References
- A. Pictet, E. Khotinsky: Über Acetylnitrat. in Chem. Ber. 40, 1907, S. 1163–1166, doi:10.1002/cber.190704001172.
- Louw, Robert "Acetyl nitrate" e-EROS Encyclopedia of Reagents for Organic Synthesis 2001, 1-2. doi:10.1002/047084289X.ra032
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.