(acyl-carrier-protein) S-malonyltransferase
In enzymology, a [acyl-carrier-protein] S-malonyltransferase (EC 2.3.1.39) is an enzyme that catalyzes the chemical reaction
- malonyl-CoA + acyl carrier protein ⇌ CoA + malonyl-[acyl-carrier-protein]
| Malonyl-CoA:ACP Transacylase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 2.3.1.39 | ||||||||
| CAS no. | 37257-17-3 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Thus, the two substrates of this enzyme are malonyl-CoA and acyl carrier protein, whereas its two products are CoA and malonyl-acyl-carrier-protein. This enzyme belongs to the family of transferases, specifically those acyltransferases transferring groups other than aminoacyl groups. This enzyme participates in fatty acid biosynthesis.
Nomenclature
The systematic name of this enzyme class is malonyl-CoA:[acyl-carrier-protein] S-malonyltransferase. Other names in common use include malonyl coenzyme A-acyl carrier protein transacylase,
- [acyl carrier protein]malonyltransferase,
- FabD,
- malonyl transacylase,
- malonyl transferase,
- malonyl-CoA-acyl carrier protein transacylase,
- malonyl-CoA:ACP transacylase,
- malonyl-CoA:AcpM transacylase,
- malonyl-CoA:acyl carrier protein transacylase,
- MAT, and
- MCAT.
Structure
Crystal Structures of FabD from E.coli [1] and Streptomyces coelicolor [2] are known and provide great insight into the catalytic mechanism of FabD. In E.Coli, FabD primarily involved in FAS pathway. However, in Streptomyces coelicolor, FabD is involved in FAS and polyketide synthase pathways. In both cases, the structures and active sites are very similar.
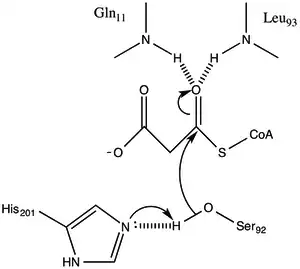
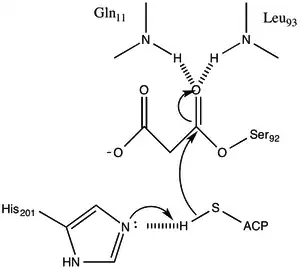
The protein has an α/β type architecture, but the fold is unique. the active site inferred from the location of the catalytic Ser92 contains a typical nucleophilic elbow as observed in α/ β hydrolases.[1] Serine 92 is hydrogen bonded to His 201 in a fashion similar to various serine hydrolases. however, instead of the carboxylic acid typically found in catalytic triads, the main chain carbonyl of Gln 250 serves as a hydrogen bond acceptor in an interaction with His 201.[1] Two other residues, Arg-117 and Glu-11 are also located in the active site, but their function is not clear.
Function
The fatty acid synthetic pathway is the principal route for the production of membrane phospholipid acyl chains in bacterial and plants.[3] The reaction sequence is carried out by a series of individual soluble proteins that are each encoded by a discrete gene, and the pathway intermediates are shuttled between the enzymes.[3] Malony-CoA:ACP Transacylase (FabD) is one such individual soluble protein and catalyzes the following reaction:
- malonyl-CoA + acyl carrier protein ⇌ CoA + malonyl-[acyl-carrier-protein]
The transfer of malonate to acyl-carrier-protein (ACP) converts the acyl groups into thioester forms which are characteristic of acyl intermediates in fatty acid synthesis and which are strictly required for the condensation reactions catalyzed by β-ketoacyl-ACP synthetase.[4]
Mechanism
Malonyl-CoA:ACP Transacylase uses a ping-pong kinetic mechanism with a bound malonyl ester as the acyl intermediate attached to a serine residue residing within a GHSLG pentapeptide.[5] FabD first binds malonyl-CoA, the malonyl moiety is then transferred to the active siteSer 92, and CoA is released from the enzyme. ACP then binds and the malonyl moiety is transferred to the terminal sulfhydryl of the ACP prosthetic group. This reaction is readily reversible.[3][6]
Industrial relevance
Among all known metabolic pathways in living systems, fatty acid biosynthesis yields the most energy dense products.[7] As a result, microbial fatty acid derivatives are emerging as a promising renewable energy alternative to fossil fuel derived transportation fuels. Recently, Khosla et al.[7] have devised a procedure to reconstitute E.Coli Fatty Acid Synthase using purified protein components (including FabD) and reported a detailed kinetic analysis of this in-vitro reconstituted system.[8] Their finding provide a new basis for assessing the scope and limitations of using E.Coli as a biocatalyst for the production of diesel fuels.
Clinical relevance
FabD as a target for Antibacterial Drug Discovery: An upcoming field
Fatty acid biosynthesis is carried out by the ubiquitous Fatty Acid Synthase.[9] Fatty acid synthase pathways are divided into two distinct molecular forms: Type I and Type II.[10] In Type I, Fatty Acid Synthase (found in humans and other mammals) is a single large polypeptide composed of several distinct domains.[11] On the other hand, each enzymatic activity (Condensation reaction, Reduction Reaction, Dehydration reaction) is found as a discrete protein in type II systems.[12] The difference in active site organization and predominance of type II FAS systems in bacteria make the enzymes of this pathway attractive targets for antibacterials.[9][12]
FabD (Acyl-Carrier-Protein S-Malonyltransferase) is a reasonable target given that a high resolution crystal structure is available.[9] However, no FabD inhibitors have been reported in the literature and review articles on this topic.[9] The simple structure and acidity of malonate seem to permit few approaches to synthesizing derivatives (acting as potential inhibitors) that retain the character of the molecule.
A second approach for using FabD as a drug target is frequently identified in the literature: FabD can provide a useful tag for locating fab genes because FabD gene is usually adjacent to at least one other fab gene.[13] However (as of 2015), no potential drugs have attempted to exploit this feature.
References
- Serre L, Verbree EC, Dauter Z, Stuitje AR, Derewenda ZS (Jun 1995). "The Escherichia coli malonyl-CoA:acyl carrier protein transacylase at 1.5-A resolution. Crystal structure of a fatty acid synthase component". The Journal of Biological Chemistry. 270 (22): 12961–4. doi:10.1074/jbc.270.22.12961. PMID 7768883.
- Keatinge-Clay AT, Shelat AA, Savage DF, Tsai SC, Miercke LJ, O'Connell JD, Khosla C, Stroud RM (Feb 2003). "Catalysis, specificity, and ACP docking site of Streptomyces coelicolor malonyl-CoA:ACP transacylase". Structure. 11 (2): 147–54. doi:10.1016/s0969-2126(03)00004-2. PMID 12575934.
- White SW, Zheng J, Zhang YM (Jun 2005). "The structural biology of type II fatty acid biosynthesis". Annual Review of Biochemistry. 74 (1): 791–831. doi:10.1146/annurev.biochem.74.082803.133524. PMID 15952903.
- Ruch FE, Vagelos PR (Dec 1973). "The isolation and general properties of Escherichia coli malonyl coenzyme A-acyl carrier protein transacylase". The Journal of Biological Chemistry. 248 (23): 8086–94. doi:10.1016/S0021-9258(19)43197-9. PMID 4584822.
- Ruch FE, Vagelos PR (Dec 1973). "The isolation and general properties of Escherichia coli malonyl coenzyme A-acyl carrier protein transacylase". The Journal of Biological Chemistry. 248 (23): 8086–94. doi:10.1016/S0021-9258(19)43197-9. PMID 4584822.
- Oefner C, Schulz H, D'Arcy A, Dale GE (Jun 2006). "Mapping the active site of Escherichia coli malonyl-CoA-acyl carrier protein transacylase (FabD) by protein crystallography". Acta Crystallographica Section D. 62 (Pt 6): 613–618. doi:10.1107/S0907444906009474. PMID 16699188.
- Liu T, Khosla C (2010). "Genetic engineering of Escherichia coli for biofuel production". Annual Review of Genetics. 44: 53–69. doi:10.1146/annurev-genet-102209-163440. PMID 20822440.
- Yu X, Liu T, Zhu F, Khosla C (Nov 2011). "In vitro reconstitution and steady-state analysis of the fatty acid synthase from Escherichia coli". Proceedings of the National Academy of Sciences of the United States of America. 108 (46): 18643–8. Bibcode:2011PNAS..10818643Y. doi:10.1073/pnas.1110852108. PMC 3219124. PMID 22042840.
- Payne DJ, Warren PV, Holmes DJ, Ji Y, Lonsdale JT (May 2001). "Bacterial fatty-acid biosynthesis: a genomics-driven target for antibacterial drug discovery". Drug Discovery Today. 6 (10): 537–544. doi:10.1016/S1359-6446(01)01774-3. PMID 11369293.
- Rock CO, Cronan JE (Jul 1996). "Escherichia coli as a model for the regulation of dissociable (type II) fatty acid biosynthesis". Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1302 (1): 1–16. doi:10.1016/0005-2760(96)00056-2. PMID 8695652.
- Chirala SS, Huang WY, Jayakumar A, Sakai K, Wakil SJ (May 1997). "Animal fatty acid synthase: functional mapping and cloning and expression of the domain I constituent activities". Proceedings of the National Academy of Sciences of the United States of America. 94 (11): 5588–93. Bibcode:1997PNAS...94.5588C. doi:10.1073/pnas.94.11.5588. PMC 20822. PMID 9159116.
- Tsay JT, Oh W, Larson TJ, Jackowski S, Rock CO (Apr 1992). "Isolation and characterization of the beta-ketoacyl-acyl carrier protein synthase III gene (fabH) from Escherichia coli K-12". The Journal of Biological Chemistry. 267 (10): 6807–14. doi:10.1016/S0021-9258(19)50498-7. PMID 1551888.
- Campbell JW, Cronan JE (2001). "Bacterial fatty acid biosynthesis: targets for antibacterial drug discovery". Annual Review of Microbiology. 55: 305–32. doi:10.1146/annurev.micro.55.1.305. PMID 11544358.
Further reading
- Alberts AW, Majerus PW, Vagelos PR (1969). "Acetyl-CoA acyl carrier protein transacylase". Methods Enzymol. Methods in Enzymology. 14: 50–53. doi:10.1016/S0076-6879(69)14009-4. ISBN 978-0-12-181871-5.
- Prescott DJ, Vagelos PR (1972). "Acyl carrier protein". Advances in Enzymology and Related Areas of Molecular Biology. Advances in Enzymology and Related Areas of Molecular Biology. Vol. 36. pp. 269–311. doi:10.1002/9780470122815.ch8. ISBN 9780470122815. PMID 4561013.
- Williamson IP, Wakil SJ (May 1966). "Studies on the mechanism of fatty acid synthesis. XVII. Preparation and general properties of acetyl coenzyme A and malonyl coenzyme A-acyl carrier protein transacylases". The Journal of Biological Chemistry. 241 (10): 2326–32. doi:10.1016/S0021-9258(18)96625-1. PMID 5330116.
- Joshi VC, Wakil SJ (Apr 1971). "Studies on the mechanism of fatty acid synthesis. XXVI. Purification and properties of malonyl-coenzyme A--acyl carrier protein transacylase of Escherichia coli". Archives of Biochemistry and Biophysics. 143 (2): 493–505. doi:10.1016/0003-9861(71)90234-7. PMID 4934182.
- Kremer L, Nampoothiri KM, Lesjean S, Dover LG, Graham S, Betts J, Brennan PJ, Minnikin DE, Locht C, Besra GS (Jul 2001). "Biochemical characterization of acyl carrier protein (AcpM) and malonyl-CoA:AcpM transacylase (mtFabD), two major components of Mycobacterium tuberculosis fatty acid synthase II". The Journal of Biological Chemistry. 276 (30): 27967–74. doi:10.1074/jbc.M103687200. PMID 11373295.
- Keatinge-Clay AT, Shelat AA, Savage DF, Tsai SC, Miercke LJ, O'Connell JD, Khosla C, Stroud RM (Feb 2003). "Catalysis, specificity, and ACP docking site of Streptomyces coelicolor malonyl-CoA:ACP transacylase". Structure. 11 (2): 147–54. doi:10.1016/S0969-2126(03)00004-2. PMID 12575934.
- Szafranska AE, Hitchman TS, Cox RJ, Crosby J, Simpson TJ (Feb 2002). "Kinetic and mechanistic analysis of the malonyl CoA:ACP transacylase from Streptomyces coelicolor indicates a single catalytically competent serine nucleophile at the active site". Biochemistry. 41 (5): 1421–7. doi:10.1021/bi012001p. PMID 11814333.