Adult stem cell
Adult stem cells are undifferentiated cells, found throughout the body after development, that multiply by cell division to replenish dying cells and regenerate damaged tissues. Also known as somatic stem cells (from Greek σωματικóς, meaning of the body), they can be found in juvenile, adult animals, and humans, unlike embryonic stem cells.
| Adult stem cell | |
|---|---|
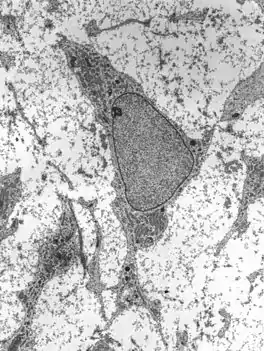 Transmission electron micrograph of an adult stem cell displaying typical ultrastructural characteristics. | |
| Details | |
| Identifiers | |
| Latin | Cellula praecursoria |
| MeSH | D053687 |
| TH | H1.00.01.0.00035 |
| Anatomical terms of microanatomy | |
Scientific interest in adult stem cells is centered around two main characteristics. The first of which is their ability to divide or self-renew indefinitely, and the second their ability to generate all the cell types of the organ from which they originate, potentially regenerating the entire organ from a few cells.[1] Unlike embryonic stem cells, the use of human adult stem cells in research and therapy is not considered to be controversial, as they are derived from adult tissue samples rather than human embryos designated for scientific research. The main functions of adult stem cells are to replace cells that are at risk of possibly dying as a result of disease or injury and to maintain a state of homeostasis within the cell.[2] There are three main methods to determine if the adult stem cell is capable of becoming a specialized cell.[2] The adult stem cell can be labeled in vivo and tracked, it can be isolated and then transplanted back into the organism, and it can be isolated in vivo and manipulated with growth hormones.[2] They have mainly been studied in humans and model organisms such as mice and rats.
Structure
Defining properties
A stem cell possesses two properties:
- Self-renewal is the ability to go through numerous cycles of cell division while still maintaining its undifferentiated state. Stem cells can replicate several times and can result in the formation of two stem cells, one stem cell more differentiated than the other, or two differentiated cells.[3]
- Multipotency or multidifferentiative potential is the ability to generate progeny of several distinct cell types, (for example glial cells and neurons) as opposed to unipotency, which is the term for cells that are restricted to producing a single cell type. However, some researchers do not consider multipotency to be essential and believe that unipotency self-renewing stem cells can exist.[4] These properties can be illustrated with relative ease in vitro, using methods such as clonogenic assays, where the progeny of a single cell is characterized. However, it is known that in vitro cell culture conditions can alter the behavior of cells, proving that a particular subpopulation of cells possesses stem cell properties in vivo is challenging, and so considerable debate exists as to whether some proposed stem cell populations in the adult are indeed stem cells.
Properties
Cell division
To ensure self-renewal, stem cells undergo two types of cell division (see Stem cell division and differentiation diagram). Symmetric division gives rise to two identical daughter stem cells, whereas asymmetric division produces one stem cell and one progenitor cell with limited self-renewal potential. Progenitors can go through several rounds of cell division before finally differentiating into a mature cell. It is believed that the molecular distinction between symmetric and asymmetric divisions lies in the differential segregation of cell membrane proteins (such as receptors) and their associated proteins between the daughter cells.[5]
Under normal conditions, tissue stem cells divide slowly and infrequently. They exhibit signs of quiescence or reversible growth arrest.[6] The niche the stem cell is found in plays a large role in maintaining quiescence.[6] Perturbed niches cause the stem cell to begin actively dividing again to replace lost or damaged cells until the niche is restored. In hematopoietic stem cells, the MAPK/ERK pathway and PI3K/AKT/mTOR pathway regulate this transition.[7] The ability to regulate the cell cycle in response to external cues helps prevent stem cell exhaustion or the gradual loss of stem cells following an altered balance between dormant and active states. Infrequent cell divisions also help reduce the risk of acquiring DNA mutations that would be passed on to daughter cells.
Plasticity
Discoveries in recent years have suggested that adult stem cells might have the ability to differentiate into cell types from different germ layers. For instance, neural stem cells from the brain, which are derived from ectoderm, can differentiate into ectoderm, mesoderm, and endoderm.[8] Stem cells from the bone marrow, which is derived from mesoderm, can differentiate into liver, lung, GI tract, and skin, which are derived from endoderm and mesoderm.[9] This phenomenon is referred to as stem cell transdifferentiation or plasticity. It can be induced by modifying the growth medium when stem cells are cultured in vitro or by transplanting them to an organ of the body different from the one they were originally isolated from. There is yet no consensus among biologists on the prevalence and physiological and therapeutic relevance of stem cell plasticity. More recent findings suggest that pluripotent stem cells may reside in blood and adult tissues in a dormant state.[10] These cells are referred to as "Blastomere Like Stem Cells" (BLSCs)[11] and "very small embryonic-like" (VSEL) stem cells, and display pluripotency in vitro.[10] As BLSCs and VSEL cells are present in virtually all adult tissues, including the lungs, brain, kidneys, muscles, and pancreas,[12] co-purification of BLSCs and VSEL cells with other populations of adult stem cells may explain the apparent pluripotency of adult stem cell populations. However, recent studies have shown that both human and murine VSEL cells lack stem cell characteristics and are not pluripotent.[13][14][15][16]
Aging
Stem cell function becomes impaired with age, and this contributes to progressive deterioration of tissue maintenance and repair.[17] A likely important cause of increasing stem cell dysfunction is an age-dependent accumulation of DNA damage in both stem cells and the cells that comprise the stem cell environment.[17] (See also DNA damage theory of aging.)
Adult stem cells can, however, be artificially reverted to a state where they behave like embryonic stem cells (including the associated DNA repair mechanisms). This was done with mice as early as 2006[18] with prospects to slow down human aging substantially.[19] Such cells are one of the various classes of induced stem cells.
Function
Signaling pathways
Adult stem cell research has been focused on uncovering the general molecular mechanisms that control their self-renewal and differentiation.
- The Notch pathway has been known to developmental biologists for decades. Its role in the control of stem cell proliferation has now been demonstrated for several cell types including hematopoietic, neural, and mammary[20] stem cells.
- These developmental pathways are also strongly implicated as stem cell regulators.[21]
- The TGFβ family of cytokines regulate the stemness of both normal and cancer stem cells.[22]
Types
Hematopoietic stem cells
Hematopoietic stem cells (HSCs) are stem cells that can differentiate into all blood cells.[23] This process is called hematopoiesis.[24] Hematopoietic stem cells are found in the bone marrow and umbilical cord blood.[25] The HSCs are generally dormant when found in adults due to their nature.[26]
Mammary stem cells
Mammary stem cells provide the source of cells for the growth of the mammary gland during puberty and gestation and play an important role in the carcinogenesis of the breast.[27] Mammary stem cells have been isolated from human and mouse tissue as well as from cell lines derived from the mammary gland. Single such cells can give rise to both the luminal and myoepithelial cell types of the gland and have been shown to have the ability to regenerate the entire organ in mice.[27]
Intestinal stem cells
Intestinal stem cells divide continuously throughout life and use a complex genetic program to produce the cells lining the surface of the small and large intestines.[28] Intestinal stem cells reside near the base of the stem cell niche, called the crypts of Lieberkuhn. Intestinal stem cells are probably the source of most cancers of the small intestine and colon.[29]
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are of stromal origin and may differentiate into a variety of tissues. MSCs have been isolated from the placenta, adipose tissue, lung, bone marrow and blood, Wharton's jelly from the umbilical cord,[30] and teeth (perivascular niche of dental pulp and periodontal ligament).[31] MSCs are attractive for clinical therapy due to their ability to differentiate, provide trophic support, and modulate innate immune response.[30] These cells differentiate into various cell types such as osteoblasts, chondroblasts, adipocytes, neuroectodermal cells, and hepatocytes.[32] Bioactive mediators that favor local cell growth are also secreted by MSCs. Anti-inflammatory effects on the local microenvironment, which promote tissue healing, are also observed. The inflammatory response can be modulated by adipose-derived regenerative cells (ADRC) including mesenchymal stem cells and regulatory T-lymphocytes. The mesenchymal stem cells thus alter the outcome of the immune response by changing the cytokine secretion of dendritic and T-cell subsets. This results in a shift from a pro-inflammatory environment to an anti-inflammatory or tolerant cell environment.[33][34]
Endothelial stem cells
Endothelial stem cells are one of the three types of multipotent stem cells found in the bone marrow. They are a rare and controversial group with the ability to differentiate into endothelial cells, the cells that line blood vessels as well as lymphatic vessels. Endothelial stem cells are an important aspect of the vascular network, even influencing the motion relating to white blood cells.
Neural stem cells
The existence of stem cells in the adult brain has been postulated following the discovery that the process of neurogenesis, the birth of new neurons, continues into adulthood in rats.[35] The presence of stem cells in the mature primate brain was first reported in 1967.[36] It has since been shown that new neurons are generated in adult mice, songbirds, and primates, including humans. Normally, adult neurogenesis is restricted to two areas of the brain – the subventricular zone, which lines the lateral ventricles, and the dentate gyrus of the hippocampal formation.[37] Although the generation of new neurons in the hippocampus is well established, the presence of true self-renewing stem cells there has been debated.[38] Under certain circumstances, such as following tissue damage in ischemia, neurogenesis can be induced in other brain regions, including the neocortex.
Neural stem cells are commonly cultured in vitro as so-called neurospheres – floating heterogeneous aggregates of cells, containing a large proportion of stem cells.[39] They can be propagated for extended periods and differentiated into both neuronal and glial cells, and therefore behave as stem cells. However, some recent studies suggest that this behavior is induced by the culture conditions in progenitor cells, the progeny of stem cell division that normally undergo a strictly limited number of replication cycles in vivo.[40] Furthermore, neurosphere-derived cells do not behave as stem cells when transplanted back into the brain.[41]
Neural stem cells share many properties with hematopoietic stem cells (HSCs). Remarkably, when injected into the blood, neurosphere-derived cells differentiate into various cell types of the immune system.[42]
Olfactory adult stem cells
Olfactory adult stem cells have been successfully harvested from the human olfactory mucosa cells, which are found in the lining of the nose and are involved in the sense of smell.[43] If they are given the right chemical environment, these cells have the same ability as embryonic stem cells to develop into many different cell types. Olfactory stem cells hold the potential for therapeutic applications and, in contrast to neural stem cells, can be harvested with ease without harm to the patient. This means they can be easily obtained from all individuals, including older patients who might be most in need of stem cell therapies.
Neural crest stem cells
Hair follicles contain two types of stem cells, one of which appears to represent a remnant of the stem cells of the embryonic neural crest. Similar cells have been found in the gastrointestinal tract, sciatic nerve, cardiac outflow tract and spinal and sympathetic ganglia. These cells can generate neurons, Schwann cells, myofibroblasts, chondrocytes, and melanocytes.[44][45]
Testicular cells
Multipotent stem cells with a claimed equivalency to embryonic stem cells have been derived from spermatogonial progenitor cells found in the testicles of laboratory mice by scientists in Germany[46][47][48] and the United States,[49][50][51][52] and, a year later, researchers from Germany and the United Kingdom confirmed the same capability using cells from the testicles of humans.[53] The extracted stem cells are known as human adult germline stem cells (GSCs)[54]
Multipotent stem cells have also been derived from germ cells found in human testicles.[55]
Clinical significance
Adult stem cell treatments have been used for many years to successfully treat leukemia and related bone/blood cancers utilizing bone marrow transplants.[56] The use of adult stem cells in research and therapy is not considered as controversial as the use of embryonic stem cells, because the production of adult stem cells does not require the destruction of an embryo.
Early regenerative applications of adult stem cells have focused on intravenous delivery of blood progenitors known as Hematopoietic Stem Cells (HSCs). CD34+ hematopoietic Stem Cells have been clinically applied to treat various diseases including spinal cord injury,[57] liver cirrhosis[58] and Peripheral Vascular disease.[59] Research has shown that CD34+ hematopoietic Stem Cells are relatively more numerous in men than in women of reproductive age group among spinal cord Injury victims.[60] Other early commercial applications have focused on Mesenchymal Stem Cells (MSCs). For both cell lines, direct injection or placement of cells into a site in need of repair may be the preferred method of treatment, as vascular delivery suffers from a "pulmonary first pass effect" where intravenously injected cells are sequestered in the lungs.[61] Clinical case reports in orthopedic applications have been published. Wakitani has published a small case series of nine defects in five knees involving surgical transplantation of mesenchymal stem cells with coverage of the treated chondral defects.[62] Centeno et al. have reported high-field MRI evidence of increased cartilage and meniscus volume in individual human clinical subjects as well as a large n=227 safety study.[63][64][65] Many other stem cell-based treatments are operating outside the US, with much controversy being reported regarding these treatments as some feel more regulation is needed as clinics tend to exaggerate claims of success and minimize or omit risks.[66]
Therapies
The therapeutic potential of adult stem cells is the focus of much scientific research, due to their ability to be harvested from the parent body that is females during the delivery.[67][68][69] In common with embryonic stem cells, adult stem cells can differentiate into more than one cell type, but unlike the former they are often restricted to certain types or "lineages". The ability of a differentiated stem cell of one lineage to produce cells of a different lineage is called transdifferentiation. Some types of adult stem cells are more capable of transdifferentiation than others, but for many there is no evidence that such a transformation is possible. Consequently, adult stem therapies require a stem cell source of the specific lineage needed, and harvesting and/or culturing them up to the numbers required is a challenge.[70][71] Additionally, cues from the immediate environment (including how stiff or porous the surrounding structure/extracellular matrix is) can alter or enhance the fate and differentiation of the stem cells.[72]
Sources
Pluripotent stem cells, i.e. cells that can give rise to any fetal or adult cell type, can be found in several tissues, including umbilical cord blood.[73] Using genetic reprogramming, pluripotent stem cells equivalent to embryonic stem cells have been derived from human adult skin tissue.[74][75][76][77] Other adult stem cells are multipotent, meaning there are several limited types of cell they can become, and are generally referred to by their tissue origin (such as mesenchymal stem cell, adipose-derived stem cell, endothelial stem cell, etc.).[78][79] A great deal of adult stem cell research has focused on investigating their capacity to divide or self-renew indefinitely, and their differentiation potential.[80] In mice, pluripotent stem cells can be directly generated from adult fibroblast cultures.[81]
Research
Cancer
In recent years, acceptance of the concept of adult stem cells has increased. There is now a hypothesis that stem cells reside in many adult tissues and that these unique reservoirs of cells not only are responsible for the normal reparative and regenerative processes but are also considered to be a prime target for genetic and epigenetic changes, culminating in many abnormal conditions including cancer.[82][83] (See cancer stem cell for more details.)
Multidrug resistance
Adult stem cells express transporters of the ATP-binding cassette family that actively pump a diversity of organic molecules out of the cell.[84] Many pharmaceuticals are exported by these transporters conferring multidrug resistance onto the cell. This complicates the design of drugs, for instance, neural stem cell-targeted therapies for the treatment of clinical depression.
Lung Organoid Model: Lung Disease in COVID-19
The virus that causes COVID-19, SARS-CoV-2, damages the lungs extensively in the presence of an overreactive immune response. Adult stem cells were extracted from deep lung biopsies and used to construct a complete lung model with both proximal and distal airway epithelia. After being developed in 3D cultures, the organoids were separated into individual cells to form 2D monolayers. These lung models were used to study the damage SARS-CoV-2 causes when applied to the apical side of the transwell.[85]
Stroke Treatment
Due to their multipotency, capacity to release growth factors, and immunomodulatory abilities, stem cell-based therapies have become a viable tool for the treatment of both acute and delayed phases of stroke. By inducing neurogenesis, angiogenesis, and synaptogenesis as well as activating endogenous restorative processes through the generation of cytokines and trophic factors, this transdifferentiation can form cells with a neural lineage.[86]
See also
References
- Mahla RS (2016). "Stem Cells Applications in Regenerative Medicine and Disease Therapeutics". International Journal of Cell Biology. 2016: 6940283. doi:10.1155/2016/6940283. PMC 4969512. PMID 27516776.
- "4. The Adult Stem Cell | stemcells.nih.gov". stemcells.nih.gov. Archived from the original on 29 July 2018. Retrieved 7 March 2021.
- "II. What are the unique properties of all stem cells? | stemcells.nih.gov". stemcells.nih.gov. Retrieved 22 February 2021.
- Mlsna L (1 January 2011). "Stem Cell Based Treatments and Novel Considerations for Conscience Clause Legislation". Indiana Health Law Review. 8 (2). doi:10.18060/2020. OCLC 54703225.
- Culurgioni S, Mari S, Bonetti P, Gallini S, Bonetto G, Brennich M, et al. (March 2018). "Insc:LGN tetramers promote asymmetric divisions of mammary stem cells". Nature Communications. 9 (1): 1025. Bibcode:2018NatCo...9.1025C. doi:10.1038/s41467-018-03343-4. PMC 5844954. PMID 29523789.
- So WK, Cheung TH (2018). "Molecular Regulation of Cellular Quiescence: A Perspective from Adult Stem Cells and Its Niches". Cellular Quiescence. Methods in Molecular Biology. Vol. 1686. pp. 1–25. doi:10.1007/978-1-4939-7371-2_1. ISBN 978-1-4939-7370-5. PMID 29030809.
- Baumgartner C, Toifl S, Farlik M, Halbritter F, Scheicher R, Fischer I, et al. (June 2018). "An ERK-Dependent Feedback Mechanism Prevents Hematopoietic Stem Cell Exhaustion". Cell Stem Cell. 22 (6): 879–892.e6. doi:10.1016/j.stem.2018.05.003. PMC 5988582. PMID 29804890.
- Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlström H, et al. (June 2000). "Generalized potential of adult neural stem cells". Science. 288 (5471): 1660–1663. Bibcode:2000Sci...288.1660C. doi:10.1126/science.288.5471.1660. PMID 10834848.
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. (May 2001). "Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell". Cell. 105 (3): 369–377. doi:10.1016/s0092-8674(01)00328-2. PMID 11348593. S2CID 11666138.
- Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ (May 2006). "A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow". Leukemia. 20 (5): 857–869. doi:10.1038/sj.leu.2404171. PMID 16498386. S2CID 24038471.
- Am Surg. 2007 Nov; 73: 1106–1110
- Zuba-Surma EK, Kucia M, Wu W, Klich I, Lillard JW, Ratajczak J, Ratajczak MZ (December 2008). "Very small embryonic-like stem cells are present in adult murine organs: ImageStream-based morphological analysis and distribution studies". Cytometry. Part A. 73A (12): 1116–1127. doi:10.1002/cyto.a.20667. PMC 2646009. PMID 18951465.
- Danova-Alt R, Heider A, Egger D, Cross M, Alt R (3 April 2012). "Very small embryonic-like stem cells purified from umbilical cord blood lack stem cell characteristics". PLOS ONE. 7 (4): e34899. Bibcode:2012PLoSO...734899D. doi:10.1371/journal.pone.0034899. PMC 3318011. PMID 22509366.
- Szade K, Bukowska-Strakova K, Nowak WN, Szade A, Kachamakova-Trojanowska N, Zukowska M, et al. (17 May 2013). "Murine bone marrow Lin⁻Sca⁻1⁺CD45⁻ very small embryonic-like (VSEL) cells are heterogeneous population lacking Oct-4A expression". PLOS ONE. 8 (5): e63329. Bibcode:2013PLoSO...863329S. doi:10.1371/journal.pone.0063329. PMC 3656957. PMID 23696815.
- Miyanishi M, Mori Y, Seita J, Chen JY, Karten S, Chan CK, et al. (6 August 2013). "Do pluripotent stem cells exist in adult mice as very small embryonic stem cells?". Stem Cell Reports. 1 (2): 198–208. doi:10.1016/j.stemcr.2013.07.001. PMC 3757755. PMID 24052953.
- Miyanishi M, Mori Y, Seita J, Chen JY, Karten S, Chan CK, et al. (August 2013). "Do pluripotent stem cells exist in adult mice as very small embryonic stem cells?". Stem Cell Reports. 1 (2): 198–208. doi:10.1016/j.stemcr.2013.07.001. PMC 3757755. PMID 24052953.
- Behrens A, van Deursen JM, Rudolph KL, Schumacher B (March 2014). "Impact of genomic damage and ageing on stem cell function". Nature Cell Biology. 16 (3): 201–207. doi:10.1038/ncb2928. PMC 4214082. PMID 24576896.
- Takahashi K, Yamanaka S (August 2006). "Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors". Cell. 126 (4): 663–676. doi:10.1016/j.cell.2006.07.024. hdl:2433/159777. PMID 16904174. S2CID 1565219.
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (November 2007). "Induction of pluripotent stem cells from adult human fibroblasts by defined factors". Cell. 131 (5): 861–872. doi:10.1016/j.cell.2007.11.019. hdl:2433/49782. PMID 18035408. S2CID 8531539.
- Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS (December 2004). "Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells". Breast Cancer Research. 6 (6): R605–R615. doi:10.1186/bcr920. PMC 1064073. PMID 15535842.
- Beachy PA, Karhadkar SS, Berman DM (November 2004). "Tissue repair and stem cell renewal in carcinogenesis". Nature. 432 (7015): 324–331. Bibcode:2004Natur.432..324B. doi:10.1038/nature03100. PMID 15549094. S2CID 4428056.
- Sakaki-Yumoto M, Katsuno Y, Derynck R (February 2013). "TGF-β family signaling in stem cells". Biochimica et Biophysica Acta (BBA) – General Subjects. 1830 (2): 2280–2296. doi:10.1016/j.bbagen.2012.08.008. PMC 4240309. PMID 22959078.
- Birbrair A, Frenette PS (April 2016). "Niche heterogeneity in the bone marrow". Annals of the New York Academy of Sciences. 1370 (1): 82–96. Bibcode:2016NYASA1370...82B. doi:10.1111/nyas.13016. PMC 4938003. PMID 27015419.
- "Medical Definition of Hematopoiesis". MedicineNet. Archived from the original on 14 March 2017. Retrieved 21 February 2020.
- "5. Hematopoietic Stem Cells [Stem Cell Information]". stemcells.nih.gov. 17 June 2001. Archived from the original on 5 June 2014. Retrieved 21 February 2020.
- Srikanth L, Sunitha MM, Venkatesh K, Kumar PS, Chandrasekhar C, Vengamma B, Sarma PV (2015). "Anaerobic Glycolysis and HIF1α Expression in Haematopoietic Stem Cells Explains Its Quiescence Nature". Journal of Stem Cells. 10 (2): 97–106. PMID 27125138. ProQuest 1769944207.
- Liu S, Dontu G, Wicha MS (June 2005). "Mammary stem cells, self-renewal pathways, and carcinogenesis". Breast Cancer Research. 7 (3): 86–95. doi:10.1186/bcr1021. PMC 1143566. PMID 15987436.
- van der Flier LG, Clevers H (March 2009). "Stem cells, self-renewal, and differentiation in the intestinal epithelium". Annual Review of Physiology. 71 (1): 241–260. doi:10.1146/annurev.physiol.010908.163145. PMID 18808327.
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, et al. (January 2009). "Crypt stem cells as the cells-of-origin of intestinal cancer". Nature. 457 (7229): 608–611. Bibcode:2009Natur.457..608B. doi:10.1038/nature07602. PMID 19092804. S2CID 4422868.
- Phinney DG, Prockop DJ (November 2007). "Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views". Stem Cells. 25 (11): 2896–2902. doi:10.1634/stemcells.2007-0637. PMID 17901396. S2CID 1352725.
- Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S (August 2005). "The efficacy of mesenchymal stem cells to regenerate and repair dental structures". Orthodontics & Craniofacial Research. 8 (3): 191–199. CiteSeerX 10.1.1.456.7504. doi:10.1111/j.1601-6343.2005.00331.x. PMID 16022721.
- Bai X, Alt E (October 2010). "Myocardial regeneration potential of adipose tissue-derived stem cells". Biochemical and Biophysical Research Communications. 401 (3): 321–326. doi:10.1016/j.bbrc.2010.09.012. PMID 20833143.
- Aggarwal S, Pittenger MF (February 2005). "Human mesenchymal stem cells modulate allogeneic immune cell responses". Blood. 105 (4): 1815–1822. doi:10.1182/blood-2004-04-1559. PMID 15494428.
- Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G (April 2007). "Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis". Arthritis and Rheumatism. 56 (4): 1175–1186. doi:10.1002/art.22511. PMID 17393437.
- Altman J, Das GD (June 1965). "Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats". The Journal of Comparative Neurology. 124 (3): 319–335. doi:10.1002/cne.901240303. PMID 5861717. S2CID 14121873.
- Lewis PD (March 1968). "Mitotic activity in the primate subependymal layer and the genesis of gliomas". Nature. 217 (5132): 974–975. Bibcode:1968Natur.217..974L. doi:10.1038/217974a0. PMID 4966809. S2CID 4169368.
- Alvarez-Buylla A, Seri B, Doetsch F (April 2002). "Identification of neural stem cells in the adult vertebrate brain". Brain Research Bulletin. 57 (6): 751–758. doi:10.1016/s0361-9230(01)00770-5. PMID 12031271. S2CID 40684602.
- Bull ND, Bartlett PF (November 2005). "The adult mouse hippocampal progenitor is neurogenic but not a stem cell". The Journal of Neuroscience. 25 (47): 10815–10821. doi:10.1523/JNEUROSCI.3249-05.2005. PMC 6725873. PMID 16306394.
- Reynolds BA, Weiss S (March 1992). "Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system". Science. 255 (5052): 1707–1710. Bibcode:1992Sci...255.1707R. doi:10.1126/science.1553558. PMID 1553558.
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A (December 2002). "EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells". Neuron. 36 (6): 1021–1034. doi:10.1016/s0896-6273(02)01133-9. PMID 12495619. S2CID 3250093.
- Marshall GP, Laywell ED, Zheng T, Steindler DA, Scott EW (March 2006). "In vitro-derived "neural stem cells" function as neural progenitors without the capacity for self-renewal". Stem Cells. 24 (3): 731–738. doi:10.1634/stemcells.2005-0245. PMID 16339644. S2CID 25223188.
- Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL (January 1999). "Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo". Science. 283 (5401): 534–537. Bibcode:1999Sci...283..534B. doi:10.1126/science.283.5401.534. PMID 9915700.
- Murrell W, Féron F, Wetzig A, Cameron N, Splatt K, Bellette B, et al. (June 2005). "Multipotent stem cells from adult olfactory mucosa". Developmental Dynamics. 233 (2): 496–515. doi:10.1002/dvdy.20360. PMID 15782416. S2CID 38561781.
- Sieber-Blum M, Hu Y (December 2008). "Epidermal neural crest stem cells (EPI-NCSC) and pluripotency". Stem Cell Reviews. 4 (4): 256–260. doi:10.1007/s12015-008-9042-0. PMID 18712509. S2CID 23267408.
- Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ (August 2002). "Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness". Neuron. 35 (4): 657–669. doi:10.1016/s0896-6273(02)00827-9. PMC 2728576. PMID 12194866.
- "Testicle cells may aid research". 25 March 2006.
- "Study: Mice Testes Act Like Stem Cells". CBS News. Associated Press. 24 March 2006.
- Weiss R (25 March 2006). "Embryonic Stem Cell Success". Washington Post.
- "Promising New Source of Stem Cells: Mouse Testes Produce Wide Range of Tissue Types". Science Daily. 24 September 2007.
- Miller B (20 September 2007). "Testicles yield stem cells in science breakthrough". ABC News. Australian Broadcasting Corporation.
- Minkel JR (19 September 2007). "Testes May Prove Fertile Source of Stem Cells". Scientific American.
- "Stem Cells in Adult Testes Provide Alternative to Embryonic Stem Cells for Organ Regeneration". Cornell University. 20 September 2007.
- Waters R (8 October 2008). "Testicle Stem Cells Become Bone, Muscle in German Experiments". Bloomberg News.
- Schultz N (9 October 2008). "A Source of Men's Stem Cells – Stem cells from human testes could be used for personalized medicine". Technology Review.
- Fox M (2 April 2006). "U.S. Firm Says It Made Stem Cells From Human Testes". Washington Post. Reuters.
- Bone Marrow Transplant Retrieved on 21 November 2008
- Srivastava A, Bapat M, Ranade S, Srinivasan V, Murugan P, Manjunath S, et al. (2010). "Multiple injections of in vitro expanded autologous bone marrow stem cells for cervical level spinal cord injury – a case report". Journal of Stem Cells & Regenerative Medicine. 6 (3): 175–176. PMID 24693165.
- Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, et al. (October 2006). "Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy". Stem Cells. 24 (10): 2292–2298. doi:10.1634/stemcells.2005-0542. PMID 16778155. S2CID 5649484.
- Subrammaniyan R, Amalorpavanathan J, Shankar R, Rajkumar M, Baskar S, Manjunath SR, et al. (September 2011). "Application of autologous bone marrow mononuclear cells in six patients with advanced chronic critical limb ischemia as a result of diabetes: our experience". Cytotherapy. 13 (8): 993–999. doi:10.3109/14653249.2011.579961. PMID 21671823. S2CID 27251276.
- Dedeepiya VD, Rao YY, Jayakrishnan GA, Parthiban JK, Baskar S, Manjunath SR, et al. (5 July 2012). "Index of CD34+ Cells and Mononuclear Cells in the Bone Marrow of Spinal Cord Injury Patients of Different Age Groups: A Comparative Analysis". Bone Marrow Research. 2012: 787414. doi:10.1155/2012/787414. PMC 3398573. PMID 22830032.
- Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. (June 2009). "Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect". Stem Cells and Development. 18 (5): 683–692. doi:10.1089/scd.2008.0253. PMC 3190292. PMID 19099374.
- Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H (January 2007). "Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees". Journal of Tissue Engineering and Regenerative Medicine. 1 (1): 74–79. doi:10.1002/term.8. PMID 18038395. S2CID 24093117.
- Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D (December 2008). "Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells". Medical Hypotheses. 71 (6): 900–908. doi:10.1016/j.mehy.2008.06.042. PMID 18786777.
- Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D (May 2008). "Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells". Pain Physician. 11 (3): 343–353. PMID 18523506.
- Centeno CJ, Schultz JR, Cheever M, Robinson B, Freeman M, Marasco W (March 2010). "Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique". Current Stem Cell Research & Therapy. 5 (1): 81–93. doi:10.2174/157488810790442796. PMID 19951252.
- "The ISSCR Releases New Guidelines to Shape Future of Stem Cell Therapy" (Press release). International Society for Stem Cell Research. 3 December 2008.
- Liao YH, Verchere CB, Warnock GL (April 2007). "Adult stem or progenitor cells in treatment for type 1 diabetes: current progress". Canadian Journal of Surgery. Journal Canadien de Chirurgie. 50 (2): 137–142. PMC 2384257. PMID 17550719.
- Mimeault M, Hauke R, Batra SK (September 2007). "Stem cells: a revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies". Clinical Pharmacology and Therapeutics. 82 (3): 252–264. doi:10.1038/sj.clpt.6100301. PMID 17671448. S2CID 12411918.
- Christoforou N, Gearhart JD (May 2007). "Stem cells and their potential in cell-based cardiac therapies". Progress in Cardiovascular Diseases. 49 (6): 396–413. doi:10.1016/j.pcad.2007.02.006. PMID 17498520.
- Raff M (November 2003). "Adult stem cell plasticity: fact or artifact?". Annual Review of Cell and Developmental Biology. 19 (1): 1–22. doi:10.1146/annurev.cellbio.19.111301.143037. PMID 14570561.
- Smith S, Neaves W, Teitelbaum S (June 2007). "Adult versus embryonic stem cells: treatments". Science. 316 (5830): 1422–1423, author reply 1422–1423. doi:10.1126/science.316.5830.1422b. PMID 17556566. S2CID 12738214.
- Huang C, Dai J, Zhang XA (June 2015). "Environmental physical cues determine the lineage specification of mesenchymal stem cells". Biochimica et Biophysica Acta (BBA) – General Subjects. 1850 (6): 1261–1266. doi:10.1016/j.bbagen.2015.02.011. PMC 4411082. PMID 25727396.
- Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M (May 2007). "A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues". Leukemia. 21 (5): 860–867. doi:10.1038/sj.leu.2404630. PMID 17344915.
- "Me too, too – How to make human embryonic stem cells without destroying human embryos". The Economist. 22 November 2007.
- Kolata G (21 November 2007). "Scientists Bypass Need for Embryo to Get Stem Cells". The New York Times.
- McIlroy A (21 November 2007). "Stem-cell method hailed as 'massive breakthrough'". Globe and Mail. Canada.
- Park A (20 November 2007). "A Breakthrough on Stem Cells". Time. Archived from the original on 21 November 2007.
- Barrilleaux B, Phinney DG, Prockop DJ, O'Connor KC (November 2006). "Review: ex vivo engineering of living tissues with adult stem cells". Tissue Engineering. 12 (11): 3007–3019. doi:10.1089/ten.2006.12.3007. PMID 17518617.
- Gimble JM, Katz AJ, Bunnell BA (May 2007). "Adipose-derived stem cells for regenerative medicine". Circulation Research. 100 (9): 1249–1260. doi:10.1161/01.RES.0000265074.83288.09. PMC 5679280. PMID 17495232.
- Gardner RL (March 2002). "Stem cells: potency, plasticity and public perception". Journal of Anatomy. 200 (Pt 3): 277–282. doi:10.1046/j.1469-7580.2002.00029.x. PMC 1570679. PMID 12033732.
- Takahashi K, Yamanaka S (August 2006). "Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors". Cell. 126 (4): 663–676. doi:10.1016/j.cell.2006.07.024. hdl:2433/159777. PMID 16904174. S2CID 1565219.
- Presnell SC, Petersen B, Heidaran M (October 2002). "Stem cells in adult tissues". Seminars in Cell & Developmental Biology. 13 (5): 369–376. doi:10.1016/s1084952102000939. PMID 12324219.
- Cogle CR, Guthrie SM, Sanders RC, Allen WL, Scott EW, Petersen BE (August 2003). "An overview of stem cell research and regulatory issues". Mayo Clinic Proceedings. 78 (8): 993–1003. doi:10.4065/78.8.993. PMID 12911047.
- Chaudhary PM, Roninson IB (July 1991). "Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells". Cell. 66 (1): 85–94. doi:10.1016/0092-8674(91)90141-k. PMID 1712673. S2CID 1717379.
- Tindle C, Fuller M, Fonseca A, Taheri S, Ibeawuchi SR, Beutler N, et al. (August 2021). Radisic M, Van der Meer JW, Clevers H (eds.). "Adult stem cell-derived complete lung organoid models emulate lung disease in COVID-19". eLife. 10: e66417. doi:10.7554/eLife.66417. PMC 8463074. PMID 34463615.
- Fernández-Susavila H, Bugallo-Casal A, Castillo J, Campos F (2019). "Adult Stem Cells and Induced Pluripotent Stem Cells for Stroke Treatment". Frontiers in Neurology. 10: 908. doi:10.3389/fneur.2019.00908. PMC 6722184. PMID 31555195.
External links
- NIH Stem Cell Information Resource, a resource for stem cell research
- Nature Reports Stem Cells Background information, research advances, and debates about stem cell science
- UMDNJ Stem Cell and Regenerative Medicineprovides educational materials and research resources
- Stem Cell Research at Johns Hopkins University