Allyl phenyl ether
Allyl phenyl ether is an organic compound with the formula C6H5OCH2CH=CH2. It is a colorless solid.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
[(Prop-2-en-1-yl)oxy]benzene | |
| Other names
(Allyloxy)benzene 3-Phenoxypropene Allyloxybenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.015.569 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H10O | |
| Molar mass | 134.178 g·mol−1 |
| Appearance | colorless solid |
| Melting point | 90 °C (194 °F; 363 K) |
| Boiling point | 191.7 °C (377.1 °F; 464.8 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H312, H332 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
Allyl phenyl ether is prepared by the reaction of sodium phenoxide with allyl bromide:[1]
- C6H5ONa + BrCH2CH=CH2 → C6H5OCH2CH=CH2 + NaBr
The yield is almost quantitative when the reaction is conducted in homogeneous solution using dimethoxyethane. When the reaction is conducted as a slurry in diethyl ether, the predominant product is, after acidic work-up, 2-allylphenol.
Reactions
Allyl phenyl ether converts to 2-allylphenol in the presence of acid catalysts. This conversion is an example of the Claisen rearrangement.[2][3]
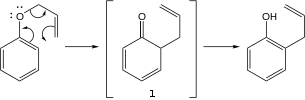
The Claisen rearrangement
References
- Kornblum, Nathan; Lurie, Arnold P. (1959). "Heterogeneity as a Factor in the Alkylation of Ambident Anions: Phenoxide Ions1,2". Journal of the American Chemical Society. 81 (11): 2705–2715. doi:10.1021/ja01520a030.
- Martín Castro, Ana M. (2004). "Claisen Rearrangement over the Past Nine Decades". Chemical Reviews. 104 (6): 2939–3002. doi:10.1021/cr020703u. PMID 15186185.
- Yadav, G. D.; Lande, S. V., UDCaT-5: A Novel and Efficient Solid Superacid Catalyst for Claisen Rearrangement of Substituted Allyl Phenyl Ethers. Synthetic Communications 2007, 37 (6), 941-946
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.