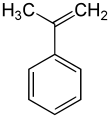
α-Methylstyrene
α-Methylstyrene (AMS) is an organic compound with the formula C6H5C(CH3)=CH2. It is a colorless oil.[4]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(Prop-1-en-2-yl)benzene | |||
| Other names
2-Phenylpropene; 2-Phenylpropylene; 1-Methyl-1-phenylethylene; 1-Methyl-1-phenylethene; 1-Phenyl-1-methylethylene; 1-Phenyl-1-methylethene; (1-Methylethenyl)benzene; β-Phenylpropene; β-Phenylpropylene; α-Methylstyrol; α-Methylvinylbenzene; Isopropenylbenzene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | AMS | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.459 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2303 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C9H10 | |||
| Molar mass | 118.179 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.91 g/cm3 | ||
| Melting point | −24 °C (−11 °F; 249 K) | ||
| Boiling point | 165 to 169 °C (329 to 336 °F; 438 to 442 K) | ||
| Insoluble | |||
| Vapor pressure | 2 mmHg (20 °C)[1] | ||
| -80.1·10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling:[2] | |||
   | |||
| Warning | |||
| H226, H319, H335, H411 | |||
| P210, P233, P240, P241, P242, P243, P261, P264+P265, P271, P273, P280, P303+P361+P353, P304+P340, P305+P351+P338, P319, P337+P317, P370+P378, P391, P403+P233, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 45 °C (113 °F; 318 K) | ||
| Explosive limits | 1.9–6.1%[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
4900 mg/kg (oral, rat)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
C 100 ppm (480 mg/m3)[1] | ||
REL (Recommended) |
TWA 50 ppm (240 mg/m3) ST 100 ppm (485 mg/m3)[1] | ||
IDLH (Immediate danger) |
700 ppm[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Synthesis and reactions
AMS is a precursor to plasticizers, resins, and polymers.[5]
AMS and acetophenone are byproducts formed in a variation of the cumene process. It can also be produced by dehydrogenation of cumene.
The homopolymer obtained from this monomer, poly(α-methylstyrene), is unstable, being characterized by a low ceiling temperature.[6]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0429". National Institute for Occupational Safety and Health (NIOSH).
- "alpha-Methylstyrene". pubchem.ncbi.nlm.nih.gov.
- "alpha-Methyl styrene". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- James, Denis H.; Castor, William M. (2007). "Styrene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_329.pub2.
- "What is alpha-methylstyrene (AMS)?". Archived from the original on 2007-02-28. Retrieved 2009-01-07.
- Stevens, Malcolm P. (1999). "6". Polymer Chemistry an Introduction (3rd ed.). New York: Oxford University Press. pp. 193–194. ISBN 978-0-19-512444-6.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.