Alpha cleavage
Alpha-cleavage (α-cleavage) in organic chemistry refers to the act of breaking the carbon-carbon bond[1] adjacent to the carbon bearing a specified functional group.[2]
Mass spectrometry
Generally this topic is discussed when covering tandem mass spectrometry fragmentation and occurs generally by the same mechanisms.[3][4]
For example, of a mechanism of alpha-cleavage, an electron is knocked off an atom (usually by electron collision) to form a radical cation. Electron removal generally happens in the following order: 1) lone pair electrons, 2) pi bond electrons, 3) sigma bond electrons.
One of the lone pair electrons moves down to form a pi bond with an electron from an adjacent (alpha) bond. The other electron from the bond moves to an adjacent atom (not one adjacent to the lone pair atom) creating a radical. This creates a double bond adjacent to the lone pair atom (oxygen is a good example) and breaks/cleaves the bond from which the two electrons were removed.
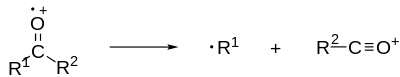 Example of alpha cleavage
Example of alpha cleavage
In molecules containing carbonyl groups, alpha-cleavage often competes with McLafferty rearrangement.
Photochemistry
In photochemistry, it is the homolytic cleavage of a bond adjacent to a specified group.[4][5]
See also
References
- Hathaway, Bruce A. (2005). Organic chemistry the easy way. Woodbury, N.Y: Barron's Educational Series. p. 315. ISBN 0-7641-2794-2.
- "α-cleavage (alpha-cleavage)". IUPAC Compendium of Chemical Terminology (Gold Book). IUPAC. Archived from the original on 20 July 2008. Retrieved 2008-07-08.
- Todd, J. F. J. (1991). "Recommendations for nomenclature and symbolism for mass spectroscopy (including an appendix of terms used in vacuum technology). (Recommendations 1991)". Pure and Applied Chemistry. 63 (10): 1541. doi:10.1351/pac199163101541.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "α-cleavage (alpha-cleavage)". doi:10.1351/goldbook.A00004
- Verhoeven, J. W. (1996). "Glossary of terms used in photochemistry (IUPAC Recommendations 1996)". Pure and Applied Chemistry. 68 (12): 2223. doi:10.1351/pac199668122223.