Aminoacetone
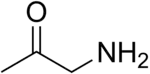
Aminoacetone is the simplest monopeptide with the formula CH3C(O)CH2NH2. Although stable in the gaseous form, once condensed it reacts with itself. The protonated derivative forms stable salts, e.g. aminoacetone hydrochloride ([CH3C(O)CH2NH3]Cl)). The semicarbazone of the hydrochloride is another bench-stable precursor.[2] Aminoacetone is a metabolite that is implicated in the biosynthesis of methylglyoxal.[3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-Aminopropan-2-one[1] | |
| Other names
Aminoacetone[1] alpha-Aminoacetone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.236.907 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H7NO | |
| Molar mass | 73.095 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 63. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- John D. Hepworth (1965). "Aminoacetone Semicarbazone Hydrochloride". Organic Syntheses. 45: 1. doi:10.15227/orgsyn.045.0001.
- Bechara, Etelvino J.H.; Dutra, Fernando; Cardoso, Vanessa E.S.; Sartori, Adriano; Olympio, Kelly P.K.; Penatti, Carlos A.A.; Adhikari, Avishek; Assunção, Nilson A. (2007). "The dual face of endogenous α-aminoketones: Pro-oxidizing metabolic weapons". Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 146 (1–2): 88–110. doi:10.1016/j.cbpc.2006.07.004. PMID 16920403.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.