Anaptychia ciliaris
Anaptychia ciliaris is a species of fruticose lichen in the family Physciaceae.[2] It is predominantly found in Northern Europe, with its range extending to European Russia, the Caucasus, Central and Southern Europe, the Canary Islands, and some parts of Asia. Initially mentioned in botanical literature by Italian botanist Fabio Colonna in 1606, the species was formally described by Carl Linnaeus in 1753, who highlighted its unique physical characteristics such as its grey colour, leafy form with linear fringe-like segments, and the presence of hair-like structures, or cilia. This lichen displays adaptability in its choice of substrates, predominantly growing on tree barks and less commonly on rocks.
| Anaptychia ciliaris | |
|---|---|
.jpg.webp) | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Fungi |
| Division: | Ascomycota |
| Class: | Lecanoromycetes |
| Order: | Caliciales |
| Family: | Physciaceae |
| Genus: | Anaptychia |
| Species: | A. ciliaris |
| Binomial name | |
| Anaptychia ciliaris | |
| Synonyms[1] | |
|
List
| |
Throughout history, the lichen has played a critical role in early scientific investigations about lichen structure and development. Early botanists like Joseph Pitton de Tournefort and Johann Hedwig made noteworthy observations about the lichen's structure. More recent studies have probed its potential biological activities, including antibacterial, insecticidal, and antioxidant properties. In addition, A. ciliaris has been used in research monitoring atmospheric pollution and detecting air pollution post the Chernobyl disaster. Beyond its scientific implications, the lichen has had various practical applications. For example, in the 17th century, it was one of several lichen ingredients in "Cyprus Powder", used as a personal grooming and cosmetic product.
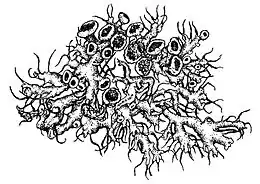
Anaptychia ciliaris can be readily identified by its sizeable stature and distinct cilia. Morphologically, it is recognized by its fruticose thallus with shades ranging from greyish-white to brownish-grey, which appears greenish when wet. It forms extensive colonies with linear structures known as laciniae. These structures branch dichotomously and are covered in fine hairs, giving a pubescent appearance. Reproductive structures known as apothecia can be found on the thallus surface, displaying characteristics ranging from laminal, stipitate, to subsessile. The unique morphology of A. ciliaris is sensitive to air pollutants, leading to observable changes in its form under adverse conditions. Two species of green algae in the genus Trebouxia have been shown to serves as the photobiont partners of this lichen.
Taxonomy

According to Annie Lorrain Smith, Anaptypia ciliaris was first mentioned in the botanical literature by the Italian botanist Fabio Colonna in his Ekphrasis (1606), a work notable for its detailed illustrations of plants using copperplate engravings.[3] It was formally described as a new species in 1753 by Carl Linnaeus, as Lichen ciliaris. Linnaeus described it as a somewhat erect, leafy, grey lichen with linear, fringe-like segments that are ciliate (having hair-like structures). He said it resembled a tree moss with notably hairy edges, and with small shield-like structures (apothecia), and cited multiple references that described the lichen in similar ways, emphasizing its larger size, hairy characteristics, and shielded appearance. Linnaeus noted that this lichen is found on trees in Europe.[4] Julius von Flotow is credited by Index Fungorum with the transfer of the taxon to the genus Anaptychia in 1850.[5] A specimen illustrated by Johann Jacob Dillenius in 1742 was selected as the lectotype.[6]
Anaptychia ciliaris is the type species of Anaptychia.[7] This genus was proposed by Gustav Wilhelm Körber in 1848 as a replacement for the name Hagenia, suggested by Franz Gerhard Eschweiler in 1824, but already being used for a plant genus. Körber described genus Anaptychia as follows (translated from Latin): "Apothecia bordered by a resupinate thallus (ascending thallus is channelled)".[8] Although he did not assign a type species himself, A. ciliaris was later designated as type by Louis Pfeiffer in 1872.[7]
Subtaxa
Several subspecies (subsp.), varieties (v.), and forms (f.) of the lichen have been described; Index Fungorum lists 45 of these subtaxa.[9] This following list is representative, but not exhaustive:
- f. ciliaris
- This is the nominate form, in which pycnidia are rare or absent, and the margins of apothecia are distinctly lacinulate (i.e., fringed or tattered).[6]
- f. agropia (Ach.) Boist. (1903)[10]
- In this form, pycnidia are rare or absent, and the margins of apothecia are crenate (scalloped) or almost entire (i.e., smooth and not lobed or toothed).[6]
- f. verrucosa (Ach.) Boist. (1903)[10]
- In this form, pycnidia are numerous; the verrucae are the same colour as the thallus or somewhat darker.[6]
- f. melanosticta (Ach.) Boberski (1886)
- In this form, pycnidia are numerous; the verrucae are dark brown to blackish brown.[6]
- f. nigrescens (Bory) Zahlbr. (1931)[11]
- In this form the laciniae less than 2 mm wide.[6]
- subspecies mamillata (Taylor) D.Hawks. & P.James (1980)[12]
- Compared to the nominate variety, subsp. mamillata has narrower lobes (about 2 mm wide), and a colouration that ranges from dark grey to brown when dry, to dark-olive green when wet, and its lacks and pruina on the thallus surface.[13]
- var. melanosticta (Ach.) Boisel (1903)[10]
- This variety is dark brown with a sparsely hairy upper surface and is mainly found on seaside rocks, especially rocks visited by birds.[14]
In 1962, Kurokawa identified five forms of Anaptychia.[6] However, by 1973, he believed these distinctions were merely character variations caused by different environmental conditions, thus deeming them taxonomically insignificant. He subsequently grouped them as synonyms.[15] This perspective is shared by Species Fungorum, which does not recognize these subtaxa as having individual taxonomic importance and categorizes them under a collective synonymy.[1] Notably, most of these classifications have not been assessed using contemporary molecular phylogenetics. One exception is A. ciliaris var. melanosticta from coastal regions. DNA analysis revealed it might be distinct enough to be considered its own species, as it emerged as a sister taxon to other tested A. ciliaris samples.[16]
Description
.jpg.webp)
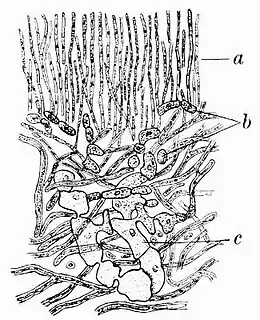
Anaptychia ciliaris has a growth form that bridges the characteristics of foliose and fruticose lichens. While it displays tendencies of both types, its classification often shifts between the two groups. The lichen has a somewhat fruticose habit, attaching to its substrate at a single base point. However, its fronds lay close to the ground, with a dorsiventral internal structure. This includes an upper "fibrous" cortex made of tightly packed, parallel hyphae and an algal layer where the photobionts are interspersed between the cortex and the medulla's looser hyphae. Its lower cortex comprises hyphae running somewhat parallel to the surface. The lichen's upper surface has a greyish hue, while the underside is either greyish or whitish.[17] It tends to be greenish, however, when wet.[18] This lichen species forms loose attachments to its substratum, contributing to the development of extensive colonies that can span up to 15 cm (6 in) or more in diameter. These colonies are characterized by elongated, linear structures that repeatedly dichotomously branch, referred to as laciniae. The laciniae generally have an upward, somewhat ascending orientation towards their tips, measuring over 2 mm (1⁄16 in) in width. They appear either flat or somewhat convex and are covered in fine hair-like structures, giving them a pubescent appearance.[6] The fibrous outer cortex imparts some strength to the laciniae, which are then resulting somewhat upright in habit.[19] Numerous pale, lateral cilia lie along the margins of the lobes.[13] They are dark grey to black, and up to 15 mm (3⁄5 in) long.[14] Sometimes, these long cilia form hapters—aerial attachment organs with highly adhesive hyphae—and after contacting something nearby induces branching and then a spreading sheath; this is quite often another lobe of its own thallus, resulting in entanglement.[20]
Beneath the laciniae, the thallus is decorticate (i.e., it lacks a cortex) and appears paler than its upper surface. The lower surface features irregular veining and is adorned with marginal rhizines, which share the same colour as the thallus. These rhizines measure between 1 and 6 mm (1⁄16 and 1⁄4 in) in length and may be either simple or occasionally branched towards the tips. The laciniae themselves are approximately 300 μm thick. The upper cortex displays irregular thickening, and its lower surface has flexuous contours, occasionally extending downward to the lower surface of the thallus without any medullary covering. The photobiont layer, housing cells measuring 10–15 μm in diameter, is frequently interrupted by the upper cortex, resulting in a discontinuous pattern. The medullary layer is typically very thin and may even be evanescent in some regions. Unlike the upper cortex, the lower cortex is absent in this lichen species.[6]
.jpg.webp)
Apothecia, the reproductive structures of Anaptychia ciliaris, are positioned on the thallus surface and exhibit laminal, stipitate, or subsessile characteristics. These structures typically range in diameter from 2 to 5 millimetres (1⁄16 to 3⁄16 in). Their margins are lacinulate, featuring ciliate lacinules along the edges. The disc of the apothecia appears brown or dark brown and may initially be covered in a white pruina, although this may disappear over time. The receptacle lacks spinules and has a hymenium measuring 150–200 μm in height, which turns blue when treated with iodine. The cortex of the receptacle is irregularly thickened and does not stain with iodine. The asci, which are cylindrical or somewhat clavate, have dimensions of about 120 by 30 μm and typically contain 4–8 spores. The spores themselves are dark brown, ellipsoid in shape with roundish tips, and are more or less constricted at the centre. They measure 17–23 by 28–43 μm in size, with thin and uniformly thickened walls.[6] In their early and undeveloped stages, the spores are oval and filled with granular and mucous substances. Over time, a central partition (the septum) forms, separating these contents, leading to the eventual formation of a two-cell state.[21]
No lichen products have been identified from Anaptychia ciliaris, and it does not react with any of the standard chemical spot tests used to help identify lichens.[6]
The morphology of A. ciliaris changes when exposed to adverse concentrations of ambient levels of air pollutants. It stops producing cilia, and the lobes become shorter and develop a warty upper surface.[22]
Photobiont
The photobiont partner of Anaptychia ciliaris was first identified as the green algal species Trebouxia arboricola.[23] This association was further affirmed in another study published the same year. This study suggested that Anaptychia species might not exhibit strong selectivity, instead integrating appropriate algae sourced from nearby lichens. The analysed specimen of A. ciliaris was collected from tree bark in close proximity to Xanthoria parietina and Pleurosticta acetabulum. Remarkably, the internal transcribed spacer ribosomal DNA of the A. ciliaris photobiont closely matched the sequences from the photobionts of these neighbouring lichens, suggesting they share the same algae.[24] Anaptychia ciliaris undergoes sexual reproduction through the production of meiotic spores. In lichens, this type of sexual reproduction means the individual symbiotic partners are dispersed separately and then come together to establish a new symbiotic relationship. This process can lead to the mixing of different genetic combinations and even allow for a change in symbiotic partners across unrelated species.[25]
In 2014, a different member of Trebouxia was shown to partner with Anaptychia ciliata. Trebouxia decolorans is a common and widespread symbiotic haploid green alga known for forming symbiotic associations with various lichen-forming fungi. Microsatellite primers, designed specifically to probe the genetic structure and diversity of this photobiont,[26] were used to elucidate the intra-thallus genetic diversity of T. decolorans. This revealed the presence of multiple, genetically distinct photobiont strains within the thalli of the fungi. Particularly in A. ciliata, the predominant source of photobiont diversity seems to be intrathalline somatic mutations, possibly due to the longer lifespan of the fungus, allowing ample time for mutations to accumulate.[25]
Similar species
The moss-dwelling African species Anaptychia ethiopica closely resembles A. ciliata, so much so that its authors consider it a sorediate version of that species. It is only known to occur in the mountains of Ethiopia at altitudes over 3,500 m (11,500 ft).[27]
Habitat and distribution

Anaptychia ciliaris is a lichen predominantly observed in Northern Europe, extending its distribution eastward to European Russia, the Caucasus,[6] and northeastern Russia (Murmansk). Its presence also spans Central and Southern Europe, the Canary Islands, and sporadic records in Asia.[29] Specimens from China have been collected from Xinjiang, Gansu, Shaanxi, and Hebei, all at altitudes over 1,800 m (5,900 ft).[30] In Turkey's Lake Abant Nature Park, A. ciliaris emerged as one of the top three lichen species in a biodiversity survey.[31] Furthermore, it is established in various regions of Morocco.[32] However, in the United Kingdom, there has been a discernible decline in its sightings. While it remains a rarity in Ireland, regions like Scotland and northeast England have classified A. ciliaris as locally extinct.[13] Historical records by Smith highlight that, around a century ago, this lichen showed a preference for trees in parklands and along cultivated roadsides.[33] In Finland, where the species is widely distributed, it is most abundant in populated areas, present in parks, alleys, and field edges, but it also occurs on seashore cliffs.[14]
Anaptychia ciliaris displays adaptability in its choice of substrates, predominantly growing on the bark of trees like Quercus rotundifolia, Q. pyrenaica, and Ulmus species. It has also been identified on Pinus sylvestris, P. nigra, and Juniperus oxycedrus.[34] Less commonly, its presence is marked on both calcareous and acidic rocks, as well as gravestones.[13] Additional sightings have been on Acer pseudoplatanus (sycamore) and Fagus sylvatica (beech) trees.[35] In Denmark, it is most commonly found on Fraxinus, followed by Tilia.[22] A noteworthy trait of A. ciliaris is its preference for diffuse light.[36]
In Poland, Anaptychia ciliaris is one of six species listed in the endangered category in the National red list.[37] In the Moscow region, located in the central part of the East European Plain, it is an uncommon species, and it is listed in the 2018 version of the Red Data Book of the Moscow Region. In this area, it is most likely to be found in birch grass-marsh forest. The relatively limited presence of the lichen in this region is attributed to the "transfer of suspended particles, carbon monoxide, nitrogen oxide and dioxide from the city of Moscow".[38] In Austria's Regional Red List, A. ciliaris is classified among the endangered lichens.[35] Although it was once a common sight in Upper Austria, the lichen's decline is attributed to the diminishing availability of its preferred substrate: old, occasionally moss-covered, well-fertilized, and dust-impregnated barks. Contemporary practices often result in the removal of trees before their bark matures to a consistency suitable for A. ciliaris.[39]
Historical North American literature has occasionally mentioned the presence of A. ciliaris, but such references are misinterpretations. In reality, North American instances refer to A. setifera, indicating that A. ciliaris is not native to the continent.[40]
Biomonitoring
Anaptychia ciliaris acts as a significant indicator in biomonitoring due to its heightened sensitivity to air pollution. A system was proposed to estimate regional SO2 emission levels in Denmark and North Germany by checking for the presence, health, and fertility of certain indicator lichens, including the widespread but sensitive A. ciliaris.[22] A similar quantitative scale was implemented in England and Wales. The scale uses zones, numbered from 0 (most polluted) to 10 (least polluted); A. ciliaris is found on eutrophied bark in areas ranked above zone 7, corresponding to an SO2 level of about 40 micrograms per cubic metre.[41] Historically prevalent in the Netherlands during the 19th century, its population witnessed a steep decline over the course of the 20th century. By that time, its presence had been confined to a few aged trees in specific locations within the country. Nevertheless, 2010 brought forth an unexpected discovery of thriving specimens on post-war ash trees in Brabant. This resurgence can likely be attributed to a combination of decreased levels of air pollution and the ongoing trends of climate warming.[42]
In the realm of environmental science, A. ciliaris offers significant utility. Its degradation of chlorophyll into phaeophytin serves as a method to detect air pollution. One of the strengths of this lichen as a biomonitor lies in its ease of identification and its adaptability to various substrates.[34] Additionally, A. ciliaris has been employed to gauge the lead and cadmium emissions from a municipal solid waste incinerator in France,[43] assess the levels of heavy metals in polluted areas of Greece,[44] and assess levels of air pollution near a lignite power plant.[45]
In Sweden, A. ciliaris has a history of being utilized for monitoring atmospheric pollution.[46] Furthermore, post the Chernobyl disaster, it played a crucial role in the biomonitoring of airborne radioactive fallout. Notably, the caesium-137 activity detected in Anaptychia ciliaris was significantly elevated, reaching levels as high as 14560 becquerels per kilogram.[47]
Uses
In the 17th Century, "Cyprus Powder" was used as a toilette powder (a fine powder, often fragranced, that was used for personal grooming and cosmetic purposes) to whiten, scent, and cleanse the hair. It was a blend of oakmoss, Anaptychia ciliaris, and species of Usnea, and was fragranced with ambergris or musk, combined with the essences of roses, jasmine, or orange blossoms.[48]
In the Erkowit region of eastern Sudan, the lichen, known locally as bakour, was mixed with other plants and burned to repel insects.[49]
Research
Historically, the lichen Anaptypia ciliaris has played a significant role in various scientific explorations of lichen structure and physiology. In the early days, Joseph Pitton de Tournefort, a French botanist, is believed to have made one of the initial observations of the lichen's distinctive large, dark-colored spores.[51] Holle, in 1849,[52] detailed the development of hyphae from lichen spores, using A. ciliaris as the subject of his study.[53] Shortly after, in 1850 and 1851, the lichen was used by German botanist Hermann Itzigsohn to show the existence of "spermagones" (conidia).[21] Following this, in 1853,[54] German botanist Julius Ferdinand Speerschneider reported his observation of the division of the lichen's photobiont cells (then referred to as gonidia) within a moist thin section of thallus.[55] The sections of the lichen he had cultivated in humid air showed that within two months the hyphae decomposed, whereas the algae not only remained healthy, but grew markedly and divided intensively. Soon after, in the centre of the decomposing section of thallus appeared small, point-like light green structures that transformed into lichen primordia.[56]
Further insights into lichen structure came when Reginald Heber Howe Jr. presented the lichen's cortex as an exemplar of the "fibrous" cortex — a tissue type characterized by long, slender, infrequently branched hyphae growing parallel to the surface.[57] The eighteenth-century work of German bryologist Johann Hedwig added depth to these findings. In his Fundamentum Historiae Naturalis Muscorum Frondosorum from 1784, he described and depicted Anaptypia ciliaris apothecia, its dark-colored septate spores, and pycnidia. He identified minute bodies associated with the organism, measuring approximately 50 μm long and 24 μm thick, as the "semina"[58] — a term which Acharius would later replace in 1803 with "spores".[59]
In the subsequent century, the study of Anaptypia ciliaris continued to evolve. In 1894, Pierre Augustin Dangeard focused on the origin and progression of asci in lichens using Anaptypia ciliaris as his specimen.[60] René Maire delved into the realm of cellular biology, observing nuclear division within an ascospore before the formation of a septum.[33] Beyond its cellular significance, the lichen's utility was also explored. In 1825, Joseph Placide Alexandre Léorier chronicled the endeavors of Roy of Tonnerre, who pioneered a technique to produce alcohol from lichens, notably Anaptypia ciliaris. This innovation was particularly remarkable given that lichens, in contrast to fruits or grains, typically lack abundant sugars typically used in traditional alcohol production.[61]
More recent research on Anaptychia ciliaris has revealed a spectrum of potential biological activities. The lichen's extracts have been assessed for antibacterial and insecticidal efficacy against specific pathogenic bacteria and the mosquito Culiseta longiareolata larvae, with the findings indicating moderate larvicidal properties.[62] Other research showed the lichen's modest antioxidant capabilities, particularly in combating oxidative stress induced by AFB1 in human lymphocytes.[63] When tested against fish bacterial pathogens, A. ciliaris demonstrated antibacterial actions, particularly towards Aeromonas hydrophila, Streptococcus agalactiae, Enterococcus faecalis, and Lactococcus garvieae.[64] Lastly, the presence and concentration of active metabolites in A. ciliaris, inclusive of numerous antioxidants, were observed to differ based on its tree-substrate.[65]
Growth in culture
The mycobiont of Anaptychia ciliaris can be cultivated in axenic (pure) culture. More than 100 years ago, Finnish phycologist Harry Warén described the fungal partner of A. ciliaris (then known as Physcia ciliaris) grown from spores, which he described as having compact mycelia and large globular cells in which erroneously thought to observe the presence of chlorophyll.[66][67] To initiate spore growth, researchers place apothecia from the lichen in proximity to the underside of an inverted petri dish; if the asci and ascospores are mature, the spores will be ejected onto the petri dish and begin germinating.[68] Expanding on these findings, subsequent research explored the growth conditions of A. ciliaris. The spores displayed a distinct dispersal pattern, often sticking to the growth medium individually–a pattern observed in other species and common within specific lichen families. Unlike many lichens that refrain from releasing spores in lab conditions, the apothecia of A. ciliaris consistently did so, suggesting indicate that the apothecia were sexually mature, and that both the collection season and laboratory conditions were conducive to sporulation. On average, these spores took six to seven days to begin germination.[69]
Furthermore, this lichen species displayed heterogeneous spore production despite having apothecia of approximately the same size, underscoring the impact of its heterothallic life cycle, which allows for cross-fertilization and genetic recombination. The study also revealed that glucose inhibited spore germination, consistent with prior observations. Additionally, the mycobiont's early growth stages benefited from the presence of carbon sources, leading to more robust filamentous development. These findings align with the known slow growth rate of lichens and their capacity for apothecia of various ages on a single thallus, contributing to genetic diversity. Various culture media influenced the lichen's morphological and metabolic characteristics, and pigmentation synthesis was linked to the availability of sugars and sugar alcohols. The study recommended optimal culturing conditions for this species, emphasizing the importance of media composition in shaping growth patterns and metabolic processes.[69]
Species interactions
Anaptychia ciliaris interacts with a range of lichenicolous organisms, which are species that live on, and often parasitize, lichens. Among the fungi that parasitize A. ciliaris is Tremella anaptychiae, a species described in 2017. This fungus yields spherical or tuberculate fruit bodies that vary in colour from cream, pinkish, to brownish or blackish and are found in regions like Italy, Macedonia, Spain (including the Canary Islands), Sweden,[29] and Greece. Meanwhile, Catillaria mediterranea, a lichen with a reduced thallus,[70] and Monodictys anaptychiae, a rare hyphomycete, are known to grow on A. ciliaris. The latter may exclusively target A. ciliaris, leading to thallus surface damage and discoloration.[71][72] In the UK, the subspecies Anaptychia ciliaris mamillata often exhibits numerous tiny black dots on its lobes due to parasitism by Stigmidium hageniae.[13] Additionally, A. ciliaris is infected by other lichenicolous fungi such as Pronectria tincta.[73]
References
- "Synonymy. Current Name: Anaptychia ciliaris (L.) Flot., Jber. schles. Ges. vaterl. Kultur 28: 119 (1850)". Species Fungorum. Retrieved 8 October 2023.
- "Anaptychia ciliaris (L.) Körb. ex A. Massal". Catalogue of Life. Species 2000: Leiden, the Netherlands. Retrieved 8 October 2023.
- Smith 1921, p. 3.
- Linnaeus, C. (1753). Species Plantarum (in Latin). Vol. 2. Stockholm: Lars Salvius. p. 1144.
- Flotow, J.V. (1850). "Lichenes florae silesiae. Zweiter Artikel" [Lichen flora of Silesia. Second article]. Jahresbericht der Schlesischen Gesellschaft für Vaterländische Kultur (in German). 28: 119.
- Kurokawa, Syo (1962). A Monograph of the Genus Anaptychia. Weinheim: J. Cramer. pp. 10–14.
- "Record Details: Anaptychia Körb., Grundriss Krypt.-Kunde: 197 (1848)". Index Fungorum. Retrieved 8 October 2023.
- Körber, Gustav Wilhelm (1848). Grundriss der Kryptogamen-Kunde. Zur Orientirung beim Studium der kryptogamischen Pflanzen, sowie zum Gebrauch bei seinen Vorlesungen [Outline of Cryptogam Science. For orientation during the study of cryptogamic plants, as well as for use in his lectures.] (in German). Breslau: Verlag von Eduard Trewendt. p. 87.
- "Search term: Anaptychia ciliaris". Index Fungorum. Retrieved 8 October 2023.
- Boistel, Alphonse (1903). Nouvelle flore des lichens pour la détermination facile des espèces sans microscope et sans réactifs [New lichen flora for easy species determination without a microscope and reagents] (in French). Vol. 2. Paris: Librairie Générale de l'enseignement. p. 49.
- Zahlbruckner, A. (1931). Catalogus Lichenum Universalis. Vol. 7. p. 714.
- Hawksworth, D.L.; James, P.W.; Coppins, B.J. (1980). "Checklist of British lichen-forming, lichenicolous and allied fungi". The Lichenologist. 12 (1): 1–115 [106]. doi:10.1017/s0024282980000035.
- Edwards, B.; Purvis, O.W. (2009). "Anaptychia Körb (1848)". In Smith, C.W.; Aptroot, A.; Coppins, B.J.; Fletcher, F.; Gilbert, O.L.; James, P.W.; Wolseley, P.A. (eds.). The Lichens of Great Britain and Ireland (2nd ed.). London: The Natural History Museum. p. 147. ISBN 978-0-9540418-8-5.
- Stenroos, Soili; Ahti, Teuovo; Lohtander, Katileena; Myllys, Leena (2011). Suomen jäkäläopas [Finnish Lichen Guide] (in Finnish). Helsinki: Kasvimuseo, Luonnontieteellinen keskusmuseo. pp. 57–58. ISBN 978-952-10-6804-1. OCLC 767578333.
- Kurokawa, S. (1973). "Supplementary notes on the genus Anaptychia". Journal of the Hattori Botanical Laboratory. 37: 563–607.
- Lohtander, Katileena; Ahti, Teuvo; Stenroos, Soili; Urbanavichus, Gennadii (2008). "Is Anaptychia monophyletic? A phylogenetic study based on nuclear and mitochondrial genes". Annales Botanici Fennici. 45 (1): 55–60. doi:10.5735/085.045.0106. S2CID 86072114.
- Smith 1921, pp. 99–100.
- Smith 1921, p. 246.
- Smith 1921, p. 103.
- Smith 1921, p. 94.
- Lindsay, W. Lauder (1856). A Popular History of British Lichens. London: Lovell Reeve. pp. 145–146.
- Søchting, Ulrik; Ramkar, Knur (1982). "The epiphytic lichen zones in rural Denmark and Schleswig-Holstein". Nordic Journal of Botany. 2 (2): 171–181. doi:10.1111/j.1756-1051.1982.tb01178.x.
- Dahlkild, Åsa; Källersjö, Mari; Lohtander, Katileena; Tehler, Anders (2001). "Photobiont diversity in the Physciaceae (Lecanorales)". The Bryologist. 104 (4): 527–536. doi:10.1639/0007-2745(2001)104[0527:pditpl]2.0.co;2. S2CID 85776368.
- Helms, Gert; Friedl, Thomas; Rambold, Gerhard; Mayrhofer, Helmut (2001). "Identification of photobionts from the lichen family Physciaceae using algal-specific ITS rDNA sequencing". The Lichenologist. 33 (1): 73–86. doi:10.1006/lich.2000.0298. S2CID 84606671.
- Dal Grande, Francesco; Alors, David; Divakar, Pradeep K.; Bálint, Miklós; Crespo, Ana; Schmitt, Imke (2014). "Insights into intrathalline genetic diversity of the cosmopolitan lichen symbiotic green alga Trebouxia decolorans Ahmadjian using microsatellite markers". Molecular Phylogenetics and Evolution. 72: 54–60. doi:10.1016/j.ympev.2013.12.010. PMID 24412431.
- Dal Grande, Francesco; Beck, Andreas; Singh, Garima; Schmitt, Imke (2013). "Microsatellite primers in the lichen symbiotic alga Trebouxia decolorans (Trebouxiophyceae)". Applications in Plant Sciences. 1 (3): 1–4. doi:10.3732/apps.1200400. PMC 4105286. PMID 25202529.
- Swinscow, T.D.V.; Krog, Hildur (1976). "The genera Anaptychia and Heterodermia in East Africa". The Lichenologist. 8 (2): 103–138. doi:10.1017/s0024282976000212. S2CID 85810241.
- Zamora, Juan Carlos; Diederich, Paul; Millanes, Ana M.; Wedin, Mats (2017). "An old familiar face: Tremella anaptychiae sp. nov. (Tremellales, Basidiomycota)" (PDF). Phytotaxa. 307 (4): 254–262. doi:10.11646/phytotaxa.307.4.3.
- Chen, Jian-bin; Wang, Da-peng (1999). "The lichen family Physciaceae (Ascomycota) in China I. The genus Anaptychia". Mycotaxon. 73: 335–342.
- Yavuz, Mustafa; Çobanoğlu, Gülşah (2018). "Lichen diversity of Gölcük Nature Park (Isparta), including new records for Turkey". Muzeul Olteniei Craiova. Oltenia. Studii şi comunicări. Ştiinţele Naturii. 34 (2): 57–66.
- Jury, S.L. (2013). "Lichens collected during the fifth "Iter Mediterraneum" in Morocco, 8–27 June, 1992" (PDF). Bocconea. 26: 145–149. doi:10.7320/bocc26.145.
- Smith 1921, p. 189.
- Manrique, E.; Redondo, F.; Izco, F. (1989). "Estimation of chlorophyll degradation into phaeophytin in Anaptychia ciliaris as a method to detect air pollution". Lazaroa. 11: 141–148. S2CID 140585913.
- Türk, R.; Hafellner, J. (1999). Niklfeld, H. (ed.). Rote Liste gefährdeter Flechten (Lichenes) Österreichs. 2. Fassung [Red List of Endangered Lichens (Lichenes) of Austria. 2nd Edition]. Rote Listen gefährdeter Pflanzen Österreichs 2 Auflage (in German). Vol. 10. Graz: Grüne Reihe des Bundesministeriums für Umwelt, Jugend und Familie. pp. 187–228.
- Smith 1921, p. 243.
- Matwiejuk, A. (2015). "Lichens of the Supraśl town (Podlasie, north-eastern Poland)". Steciana. 19 (3): 133–142. doi:10.12657/steciana.019.015.
- Chernenkova, Tatiana V.; Belyaeva, Nadezhda G.; Suslova, Elena G.; Aristarkhova, Ekaterina A.; Kotlov, Ivan P. (2023). "Patterns of the red-listed epiphytic species distribution in coniferous-deciduous forests of the Moscow Region". Geography, Environment, Sustainability. 16 (1): 119–131. doi:10.24057/2071-9388-2022-101. S2CID 258045764.
- Türk, Roman; Wittmann, Helmut; Pilsl, Peter (1982). "Ergebnisse der floristischen Flechtenkartierung in Oberösterreich – ein erster Überblick" [Results of the floristic lichen mapping in Upper Austria – A first overview] (PDF). Stapfia (in German). 10: 121–137.
- Lewis, Christopher J. (2019). "Heterodermia leucomela (L.) Poelt discovered in Ontario, Canada for the first time in over 150 years". Evansia. 36 (2): 30–38. doi:10.1639/0747-9859-36.2.30. S2CID 209308328.
- Gilbert, O.P. (1973). "Lichens and air pollution". In Ahmadjian, Vernon; Hale, Mason (eds.). The Lichens. New York/London: Academic Press. pp. 466–467. ISBN 978-0-12-044950-7.
- Aptroot, André (2011). "Wimpermos (Anaptychia ciliaris) vestigt zich weer in Nederland" [Wimper Moss (Anaptychia ciliaris) re-establishes itself in the Netherlands]. Buxbaumiella (in Dutch). 88: 26–27.
- Gombert, S.; J., Asta (1998). "The effect of refure incinerator fumes on the lead and cadmium content of experimentally exposed corticolous lichens". Water, Air, & Soil Pollution. 104: 29–40. Bibcode:1998WASP..104...29G. doi:10.1023/A:1004965131303. S2CID 97975435.
- Sawidis, T.; Chettri, M.K.; Zachariadis, G.A.; Stratis, J.A.; Seaward, M.R.D. (1995). "Heavy metal bioaccumulation in lichens from Macedonia in northern Greece". Toxicological & Environmental Chemistry. 50 (1–4): 157–166. doi:10.1080/02772249509358211.
- Riga-Karandinos, A.N; Karandinos, M.G (1998). "Assessment of air pollution from a lignite power plant in the plain of Megalopolis (Greece) using as biomonitors three species of lichens; impacts on some biochemical parameters of lichens". Science of the Total Environment. 215 (1–2): 167–183. Bibcode:1998ScTEn.215..167R. doi:10.1016/s0048-9697(98)00119-3.
- Borg, Lars; Aronsson, Margita (2004). "Population size and ecology of Pleurosticta acetabulum, Anaptychia ciliaris and Ramalina fraxinea in avenues and churchyards in Kalmar County, SE Sweden". Graphis Scripta. 16 (3): 37–42.
- Papastefanou, C.; Manolopoulou, M.; Sawidis, T. (1989). "Lichens and mosses: Biological monitors of radioactive fallout from the Chernobyl reactor accident". Journal of Environmental Radioactivity. 9 (3): 199–207. doi:10.1016/0265-931x(89)90044-1.
- Shukla, Ila; Azmi, Lubna; Gautam, Arti; Shukla, Shashi Kant; Rao, ChV (2018). "Lichens are the next promising candidates for medicinally active compounds". International Journal of Phytopharmacy. 8 (4): 31–38. S2CID 249961675.
- Crawford, Stuart D. (2019). "Lichens used in traditional medicine". In Ranković, Branislav (ed.). Lichen Secondary Metabolites. Bioactive Properties and Pharmaceutical Potential (2 ed.). Springer Nature Switzerland. p. 67. ISBN 978-3-030-16813-1.
- Baur, Wilhelm (1904). "Untersuchungen über die Entwickelungsgeschichte der Flechtenapothecien I." [Studies on the developmental history of lichen apothecia I.]. Botanische Zeitung (in German). 62: 21–44.
- Smith 1921, p. 155.
- Holle, G. von (1849). Zur Entwicklungsgeschichte von Borrera ciliaris. Inaugural- Dissertation [On the Developmental history of Borrera ciliaris. Inaugural Dissertation]. Gottingen: Huth.
- Smith 1921, p. 46.
- Speerschneider, J. (1853). "Zur Entwickelungsgeschichte der Hagenia ciliaris" [On the developmental history of Hagenia ciliaris]. Botanische Zeitung (in German). 11: 705–711, 721–730.
- Smith 1921, p. 24.
- Lorch, Jacob (1988). "The true nature of lichens – a historical survey". In Galun, Margalith (ed.). CRC Handbook of Lichenology. Vol. 1. Boca Raton, Florida: CRC Press. pp. 12–13. ISBN 978-0-429-29178-4.
- Smith 1921, p. 85.
- Smith 1921, p. 156.
- Smith 1921, p. 184.
- Smith 1921, p. 185.
- Smith 1921, p. 411.
- Cetin, O.T.; Akarsu, M.; Burunkaya, E.; Kesmez, O.; Arpac, E.; Cetin, H. (2013). "Mosquito larvicidal and antibacterial activities of different solvent extracts of Anaptychia ciliaris subsp. ciliaris". Egyptian Journal of Biological Pest Control. 23 (2): 287–290.
- Anar, M.; Aslan, A.; Agar, G.; Ozgencli, I. (2016). "Antigenotoxic and antioxidant activity of lichens Anaptychia ciliaris, Bryoria fuscescens, Parmotrema chinensa and Xanthoria candelaria: An in vitro study". Medicinal & Aromatic Plants. 5 (2): 1–6.
- Tas, I.; Yildirim, A.B.; Ozyigitoglu, G.C.; Turker, H.; Turker, A.U. (2019). "Lichens as a promising natural antibacterial agent against fish pathogens". Bulletin of the European Association of Fish Pathologists. 39 (1): 40–48.
- Gulnara, Badridze; Chkhubianishvili, Eva; Rapava, Luara; Kikvidze, Medea; Chigladze, Lali; Tsiklauri, Nino; Tsilosani, Ketevan; Kupradze, Inga; Chanishvili, Shota (2019). "Active metabolites of some lichens growing in Georgia". Journal of Biodiversity and Environmental Sciences. 15 (6): 1–15.
- Warén, Harry (1920). "Reinkulturen von Flechtengonidien" [Pure cultures of lichen gonidia]. Öfversigt af Finska vetenskaps-societetens förhandlingar (in German). 61: 1–79.
- Warén, H. (1921). "Beobachtungen bei Kultur von Flechtenhyphen" [Observations on the culture of lichen hyphae]. Öfversigt af Finska vetenskaps-societetens förhandlingar (in German). 62: 1–9.
- Crittenden, P.D.; David, J.C.; Hawksworth, D.L.; Campbell, F.S. (1995). "Attempted isolation and success in the culturing of a broad spectrum of lichen-forming and lichenicolous fungi". New Phytologist. 130 (2): 267–297. doi:10.1111/j.1469-8137.1995.tb03048.x.
- Molina, M. Carmen; Divakar, Pradeep K.; González, Natalia (2015). "Success in the isolation and axenic culture of Anaptychia ciliaris (Physciaceae, Lecanoromycetes) mycobiont". Mycoscience. 56 (4): 351–358. doi:10.1016/j.myc.2014.10.003.
- Brackel, Wolfgang von; Döbbeler, Peter (2020). "An addition to the knowledge of lichenicolous fungi of Greece with a key to the lichenicolous fungi on Collema s. l.". Folia Cryptogamica Estonica. 57: 147–152. doi:10.12697/fce.2020.57.13. S2CID 243640230.
- Hawksworth, D.L. (1994). "Notes on British lichenicolous fungi: VII". The Lichenologist. 26 (4): 337–347. doi:10.1006/lich.1994.1028. S2CID 83611307.
- Wedin, Mats (1993). "Concentric bodies in conidia of Monodictys anaptychiae (Hyphomycetes)". The Lichenologist. 25 (2): 203–206. doi:10.1006/lich.1993.1027. S2CID 84566776.
- Diederich, Paul; Lawrey, James D.; Ertz, Damien (2018). "The 2018 classification and checklist of lichenicolous fungi, with 2000 non-lichenized, obligately lichenicolous taxa". The Bryologist. 121 (3): 340–425 [382]. doi:10.1639/0007-2745-121.3.340. S2CID 92396850.
Cited literature
- Smith, Annie Lorrain (1921). Lichens. Cambridge Botanical Handbooks. London: Cambridge University Press.