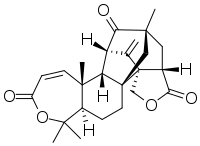
Anditomin
Anditomin is an oxygenated meroterpenoid produced by Aspergillus stellatus[1] or Aspergillus variecolor.[2]
 | |
| Names | |
|---|---|
| IUPAC name
(2S,3R,9R,12S,14R,16S,20S)-3,8,8,14-tetramethyl-21-methylidene-7,18-dioxahexacyclo[12.7.1.02,12.03,9.012,20.016,20]docos-4-ene-6,17,22-trione | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C25H30O5 | |
| Molar mass | 410.510 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Aspergillus variecolor produces anditomin from 3,5-dimethylorsellinic acid and farnesyl pyrophosphate using 12 enzymes.[3] The enzyme AndA firstly dehydrogenates one ring of preandiloid B to make preaniloid C. Next it causes a complex ring isomerisation to make anditomin.[4]
Properties
Anditomin can dissolve in ethyl acetate or chloroform. Anditomin forms tetragonal crystals with space group P41 (No. 76), with unit cell dimensions a = 9.310 and c = 24.84 Å, and unit cell volume = 2153 Å3 with Z = 4, and density = 1.27 gcm−3.[2]
References
- Chen, A.J.; Frisvad, J.C.; Sun, B.D.; Varga, J.; Kocsubé, S.; Dijksterhuis, J.; Kim, D.H.; Hong, S.-B.; Houbraken, J.; Samson, R.A. (1 June 2016). "Aspergillus section Nidulantes (formerly Emericella): Polyphasic taxonomy, chemistry and biology". Studies in Mycology. 84 (1): 1–118. doi:10.1016/j.simyco.2016.10.001. PMC 5198626. PMID 28050053.
- Simpson, Thomas J.; Walkinshaw, Malcolm D. (1981). "Anditomin, a new C25 metabolite from Aspergillus variecolor". Journal of the Chemical Society, Chemical Communications (17): 914. doi:10.1039/C39810000914.
- Matsuda, Yudai; Wakimoto, Toshiyuki; Mori, Takahiro; Awakawa, Takayoshi; Abe, Ikuro (29 October 2014). "Complete Biosynthetic Pathway of Anditomin: Nature's Sophisticated Synthetic Route to a Complex Fungal Meroterpenoid". Journal of the American Chemical Society. 136 (43): 15326–15336. doi:10.1021/ja508127q. PMID 25216349.
- Nakashima, Yu; Mitsuhashi, Takaaki; Matsuda, Yudai; Senda, Miki; Sato, Hajime; Yamazaki, Mami; Uchiyama, Masanobu; Senda, Toshiya; Abe, Ikuro (1 August 2018). "Structural and Computational Bases for Dramatic Skeletal Rearrangement in Anditomin Biosynthesis". Journal of the American Chemical Society. 140 (30): 9743–9750. doi:10.1021/jacs.8b06084. PMID 29972643. S2CID 207192801.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.