Apaziquone
Apaziquone (tentative trade name EOquin)[1] is an indolequinone that is a bioreductive prodrug similar to the older chemotherapeutic agent mitomycin C. In hypoxic cells, such as those on the inner surface of the urinary bladder, apaziquone is converted to active metabolites by intracellular reductases. The active metabolites alkylate DNA and lead to apoptosis.[2] This activity is preferentially expressed in neoplastic cells.
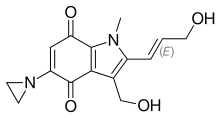 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C15H16N2O4 |
| Molar mass | 288.303 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
After administration of apaziquone directly into the urinary bladder (intravesically), the drug and its active metabolite were not detected in plasma, and there were no systemic side effects.[3][4]
Apaziquone has been applied in clinical studies sponsored by Spectrum Pharmaceuticals and Allergan for the treatment of superficial (non-muscle invasive) bladder cancer.[3] Approximately 70% of all newly diagnosed patients with bladder cancer have non-muscle invasive bladder cancer and over one million patients in the United States and Europe are affected by the disease. The U.S. Food and Drug Administration (FDA) has granted Fast Track review status to apaziquone for this indication.[5]
References
- "UvA researcher develops new bladder cancer medication". University of Amsterdam. 25 Jul 2007. Archived from the original on 2011-07-16.
- NCI. "apaziquone". Archived from the original on 9 May 2009. Retrieved 2009-06-07.
- Puri R, Palit V, Loadman PM, et al. (October 2006). "Phase I/II pilot study of intravesical apaziquone (EO9) for superficial bladder cancer". J. Urol. 176 (4 Pt 1): 1344–8. arXiv:1506.05421. doi:10.1016/j.juro.2006.06.047. PMID 16952628.
- Hendricksen K, Gleason D, Young JM, et al. (July 2008). "Safety and side effects of immediate instillation of apaziquone following transurethral resection in patients with nonmuscle invasive bladder cancer". J. Urol. 180 (1): 116–20. doi:10.1016/j.juro.2008.03.031. PMID 18485407.
- "FDA Designates Fast Track Status For Apaziquone (EOquin) For Bladder Cancer". Medical News Today. 22 Jul 2009.