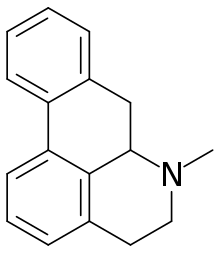
Aporphine
Aporphine is an alkaloid with the chemical formula C17H17N. It is the core chemical substructure of the aporphine alkaloids, a subclass of quinoline alkaloids. It can exist in either of two enantiomeric forms, (R)-aporphine and (S)-aporphine.
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H17N |
| Molar mass | 235.330 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Derivatives
Many different derivatives have been isolated from plants.[1] For example, many water-lilies (Nymphaea species) produce aporphine alkaloids such as nymphaeine, nymphaline, nupharine, α- and β-nupharidine.[2]
In vitro tests of some aporphine derivatives isolated from Cassytha filiformis, namely actinodaphnine, cassythine, and dicentrine, showed antiparasitic activity against Trypanosoma brucei. Investigation of possible mechanisms revealed that the compounds bind to DNA and act as intercalating agents, besides inhibiting topoisomerase activity.[3]
Aporphine natural products occur with either the (R)- or (S)-stereochemistries, or they can be achiral. Furthermore, morphine-based natural products can be heated in acid to give aporphine degradation products, like the FDA-approved Parkinson's drug apomorphine, which was first discovered by the Finnish chemist Arppe in 1845.[4]

Apomorphine
A specific type of aporphine is apomorphine. The compound is historically a morphine decomposition product made by boiling morphine with concentrated acid, hence the -morphine suffix. Contrary to its name however, apomorphine doesn’t actually contain morphine or its skeleton, nor does it bind to opioid receptors. The apo- prefix indicates that it is a morphine derivative.
Historically, apomorphine has been tried for a variety of uses, including as a way to relieve anxiety and craving in alcoholics, an emetic (a vomit-inducer), for treating stereotypies (repeated behaviour) in farmyard animals, and more recently in treating erectile dysfunction. It was also used as a private treatment of heroin addiction, but there is no clinical evidence that apomorphine is an effective and safe treatment for opiate addiction.
Currently, apomorphine is used in the treatment of Parkinson’s disease. It is a potent emetic and should not be administered without an antiemetic such as domperidone. The emetic properties of apomorphine are exploited in veterinary medicine to induce therapeutic emesis (vomiting) in canines that have recently ingested toxic or foreign substances.[5]
Effects
Aporphine is a dopamine receptor agonist, specifically D1 and D2.[6] In rodents, apomorphine administration has been demonstrated to activate gene expression specifically in the nuclei of the hypothalamus, resulting in stereotypical behaviour of erection and yawning. In humans, apomorphine produces nonsexual erections that are enhanced by erotic stimulation without changes in libido (sexual desire), but significant side effects (especially nausea) can occur. A sublingual (under the tongue) formulation of apomorphine (2 and 4 mg) with a rapid onset of action (15 to 25 min) has been developed and proven to be efficacious in ED (erectile dysfunction) patients with controlled diabetes, hypertension (high blood pressure), benign prostatic hypertrophy (the non-harmful enlargement of the prostate from the increase in size of its cells) or coronary vascular disease (disease of the arteries surrounding and supplying the heart).[7]
Synthesis
Aporphine can be synthesized in a seven-step reaction. First, 1-Benzyl-2-tosyl-1,2,3,4-tetrahydroisoquinolin-7-ylpyridine-2-sulfonate is converted to 6-Tosyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolin-1-yl-pyridine-2-sulfonate via an aryl-aryl dehydrogenative coupling reaction. Then, 6-Tosyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolin-1-yl-pyridine-2-sulfonate is reacted to 6-Tosyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolin-1-ol in a reduction reaction. After this second step, 6-Tosyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolin-1-yl Trifluoromethanesulfonate is created by swapping the hydroxyl group of 6-Tosyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolin-1-ol for a trifluoromethanesulfonate group. This 6-Tosyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolin-1-yl Trifluoromethanesulfonate is then further reduced by palladium acetate, leading to 6-Tosyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolone. The sixth step is conversion of 6-Tosyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolone to 5,6,6a,7-Tetrahydro-4H-dibenzo[de,g]quinolone in reduction reaction using samarium(II)iodide. The seventh and final step is the reductive amination of 5,6,6a,7-Tetrahydro-4H-dibenzo[de,g]quinolone to yield aporphine.[8]

A number of natural products semisynthetic analogs belonging to the aporphine class have been synthesized. These include apomorphine by Neumeyer[9] and Raminelli,[10] Pukateine by Happel,[11] Isocorydine by Di,[12] Nuciferine and Oliveroline by Cuny,[13][14] Glaucine by Meyers,[15] Dicentrine by Cava,[16] and Lysicamine by Raminelli,[17] and an overview of some of the synthetic approaches toward the aporphine ring system is outlined in the figure at the right.
Toxicity
Most aporphine alkaloids are toxic. They exhibit antagonistic effects to dopamine. Many of them have anticonvulsant activity or induced convulsions in animals and cytotoxic (toxic to cells) activity.[18]
Some aporphine alkaloids (such as crebanine) have been found to present arrhythmic activity (irregularities in the heartbeat) and also higher toxicity. In a study, a couple of target derivatives were evaluated for their antiarrhythmic potential in the mouse model of ventricular fibrillation (VF, a dangerous type of arrhythmia). Here, preliminary structure-activity/toxicity relationship analyses were carried out. Of these target derivatives, a certain bromo-substituted product of crebanine displayed significant antiarrhythmic activity and a lower toxicity. In a significant number of rats, this product caused reduction in the incidence of VF, increase in the resumption of sinus rhythm (normal heartbeat) from arrhythmia, and increase in maintaining sinus rhythm. This indicates that this specific aporphine alkaloid could be considered as a promising candidate in the treatment of arrhythmia.[19]
Pharmacology
According to the U.S. Patent & Trademark Office, aporphine derivatives can be used for treating oxidative stress induced diseases. Aporphine that can inhibit lipid peroxidase and perform the free radical scavenging activities cause protection of blood vessel smooth muscle cells. This reduces oxidative stress which may induce diseases such as cardiovascular disease, Alzheimer’s disease, kidney disease, diabetes, cancer etc.[20]
Aporphine alkaloids present in Litsea glutinosa, a tropical plant with antioxidant and anti-parasitic properties, claims to contribute to anti-cancer activity. The study of Chi P. Ndi et al., (2016) illustrates the antiproliferative and cytotoxic effects of aporphine-containing extracts of Litsea glutinosa. In silico measurements show that the 1,2-methylenedioxy group of aporphine can be utilized in the same manner as the anticancer drug etoposide.[21]
(R)-Aporphine is a dopamine receptor D1 antagonist with a Ki of 717 nM[22] and a dopamine receptor D2 antagonist with a Ki of 527 nM.[23] Aporphine and its related alkaloids bulbocapnine, boldine, glaucine, and corytuberine are antipsychotic, exert naloxone-reversible antinociceptive activity, and with the exception of corytuberine are anticonvulsant.[24] Some derivatives of aporphine such as (S)-(+)-N-propylnorapomorphine have potential as low side effect profile antipsychotics. (S)-(+)-N-Propylnorapomorphine is highly selective for meso-limbic dopaminergic tracts and function as efficacious partial agonists, with no elevation in prolactin.[25]
Pharmacokinetics
Aporphine is hydroxylated in the body to form apomorphine.[26]
Psychoactive effects
The Nymphea species (Nymphaea caerulea), as mentioned in ‘Derivatives’, is commonly used in society.[27] Its plant extracts can be ingested or vaped. Intake of Nymphaea at high doses is known to produce euphoria and hallucinations. This plant, also called the blue lotus, is sold in several forms such as dried plant material, teas, or as extract for electronic cigarettes. The psychoactive effect of the flower is due to two aporphine alkaloids; apomorphine and nuciferine. Apomorphine is known to treat diseases such as depression, schizophrenia, Parkison’s disease and erectile dysfunction. Nuciferine is used as an antipsychotic and in treatment of alcohol use disorder. The compound has mixed effects at serotonin and dopamine receptors causing the compound to be a dopaminergic agonist (it acts the same as dopamine).[28]
Effects on animals
There are no studies on aporphine specifically in animals. Studies on subcutaneous apomorphine injection are the closest thing as apomorphine is the bioactive form of aporphine. In a 5-day study, mice were given up to 10 mg/kg apomorphine subcutaneously daily. No adverse effects were observed other than a slight increase in dopamine levels.[29] However, apomorphine is used in veterinary clinics as an emetic, due to severe off-target effects that lead to vomiting.[30]
In another study, mice were given a single 40 mg/kg dose of apomorphine. Slight DNA damage was observed in brain tissue three hours after treatment.[31]
See also
References
- Stévigny C, Bailly C, Quetin-Leclercq J (March 2005). "Cytotoxic and antitumor potentialities of aporphinoid alkaloids". Current Medicinal Chemistry. Anti-Cancer Agents. 5 (2): 173–182. doi:10.2174/1568011053174864. hdl:2078.1/10136. PMID 15777224.
- Oliver-Bever B (January 1983). "Medicinal plants in tropical West Africa. II. Plants acting on the nervous system". Journal of Ethnopharmacology. 7 (1): 1–93. doi:10.1016/0378-8741(83)90082-X. PMID 6132025.
- Hoet S, Stévigny C, Block S, Opperdoes F, Colson P, Baldeyrou B, et al. (May 2004). "Alkaloids from Cassytha filiformis and related aporphines: antitrypanosomal activity, cytotoxicity, and interaction with DNA and topoisomerases". Planta Medica. 70 (5): 407–413. doi:10.1055/s-2004-818967. PMID 15124084.
- Auffret M, Drapier S, Vérin M (June 2018). "The Many Faces of Apomorphine: Lessons from the Past and Challenges for the Future". Drugs in R&D. 18 (2): 91–107. doi:10.1007/s40268-018-0230-3. PMC 5995787. PMID 29546602.
- Guardia J, Casas M, Prat G, Trujols J, Segura L, Sánchez-Turet M (October 2002). "The apomorphine test: a biological marker for heroin dependence disorder?". Addiction Biology. 7 (4): 421–426. doi:10.1080/1355621021000006206. PMID 14578019. S2CID 32386793.
- Goldman ME, Kebabian JW (January 1984). "Aporphine enantiomers. Interactions with D-1 and D-2 dopamine receptors". Molecular Pharmacology. 25 (1): 18–23. PMID 6231468.
- Anastasiadis AG, Droggin D, Davis AR, Salomon L, Shabsigh R (January 2004). "Male and Female Sexual Dysfunction: Epidemiology, Pathophysiology, Classifications, and Treatment.". Principles of Gender-Specific Medicine: Apomorphine SL. Academic Press. pp. 573–585. doi:10.1016/B978-012440905-7/50321-2.
- Su C, Xu WH, Guo RL, Zhang XL, Zhu XQ, Gao YR, Wang YQ (October 2021). "Synthesis of Aporphine Analogues via Palladium-Catalyzed Intramolecular Aryl-Aryl Dehydrogenative Coupling". The Journal of Organic Chemistry. 86 (19): 13618–13630. doi:10.1021/acs.joc.1c01649. PMID 34498883. S2CID 237455845.
- Neumeyer JL, Neustadt BR, Oh KH, Weinhardt KK, Boyce CB, Rosenberg FJ, Teiger DG (November 1973). "Aporphines. 8. Total synthesis and pharmacological evaluation of (plus or minus)-apomorphine, (plus or minus)-apocodeine, (plus or minus)-N-n-propylnorapomorphine, and (plus or minus)-N-n-propylnorapocodeine". Journal of Medicinal Chemistry. 16 (11): 1223–1228. doi:10.1021/jm00269a601. PMID 4201182.
- Muraca AC, Perecim GP, Rodrigues A, Raminelli C (2017-06-20). "Convergent Total Synthesis of (±)-Apomorphine via Benzyne Chemistry: Insights into the Mechanisms Involved in the Key Step". Synthesis. 49 (16): 3546–3557. doi:10.1055/s-0036-1588855. ISSN 0039-7881.
- Zymalkowski F, Happel KH (September 1969). "[The total synthesis of (plus minus)-pukatein]". Chemische Berichte. 102 (9): 2959–2966. doi:10.1002/cber.19691020910. PMID 5806148.
- Zhong M, Jiang Y, Chen Y, Yan Q, Liu J, Di D (2015-11-01). "Asymmetric total synthesis of (S)-isocorydine". Tetrahedron: Asymmetry. 26 (20): 1145–1149. doi:10.1016/j.tetasy.2015.09.008. ISSN 0957-4166.
- Cuny GD (2004-02-10). "Intramolecular ortho-Arylation of Phenols Utilized in the Synthesis of the Aporphine Alkaloids (.+-.)-Lirinidine and (.+-.)-Nuciferine". ChemInform. 35 (6). doi:10.1002/chin.200406170. ISSN 0931-7597.
- Ku AF, Cuny GD (March 2015). "Synthetic studies of 7-oxygenated aporphine alkaloids: preparation of (-)-oliveroline, (-)-nornuciferidine, and derivatives". Organic Letters. 17 (5): 1134–1137. doi:10.1021/acs.orglett.5b00007. PMID 25710592.
- Gottlieb L, Meyers AI (October 1990). "An asymmetric synthesis of aporphine and related alkaloids via chiral formamidines. (+)-glaucine, (+)-homoglaucine, and (-)-8,9-didemethoxythalisopavine". The Journal of Organic Chemistry. 55 (21): 5659–5662. doi:10.1021/jo00308a029. ISSN 0022-3263.
- Cava MP, Stern P, Wakisaka K (1973-01-01). "An improved photochemical aporphine synthesis: New syntheses of dicentrine and cassameridine". Tetrahedron. 29 (15): 2245–2249. doi:10.1016/S0040-4020(01)93344-7. ISSN 0040-4020.
- Rossini AF, Muraca AC, Casagrande GA, Raminelli C (October 2015). "Total Syntheses of Aporphine Alkaloids via Benzyne Chemistry: An Approach to the Formation of Aporphine Cores". The Journal of Organic Chemistry. 80 (20): 10033–10040. doi:10.1021/acs.joc.5b01634. PMID 26375603.
- Wu YC (2006). "New research and development on the Formosan Annonaceous plants. Aporphinoids". Studies in natural products chemistry. Elsevier. pp. 957–1023.
- US EPA National Center for Environmental Assessment (2009-03-15). "Synthesis and Structure-Activity Relationships of a Series of Aporphine Derivatives with Antiarrhythmic Activities and Acute Toxicity". hero.epa.gov. Retrieved 2022-03-17.
- "Broad US patent issued to Dyadic International". Focus on Catalysts. 2012 (11): 7. November 2012. doi:10.1016/s1351-4180(12)70458-0. ISSN 1351-4180.
- Chawra HS, Gupta G, Singh SK, Pathak S, Rawat S, Mishra A, Gilhotra RM (2021-11-30). "Phytochemical constituents, Ethno medicinal properties and Applications of Plant: Litsea glutinosa (Lour.) C.B. Robinson (Lauraceae)". Research Journal of Pharmacy and Technology: 6113–6118. doi:10.52711/0974-360x.2021.01062. ISSN 0974-360X.
- Hedberg MH, Linnanen T, Jansen JM, Nordvall G, Hjorth S, Unelius L, Johansson AM (August 1996). "11-substituted (R)-aporphines: synthesis, pharmacology, and modeling of D2A and 5-HT1A receptor interactions". Journal of Medicinal Chemistry. 39 (18): 3503–3513. doi:10.1021/jm960189i. PMID 8784448.
- Linnanen T, Brisander M, Unelius L, Rosqvist S, Nordvall G, Hacksell U, Johansson AM (April 2001). "Atropisomeric derivatives of 2',6'-disubstituted (R)-11-phenylaporphine: selective serotonin 5-HT(7) receptor antagonists". Journal of Medicinal Chemistry. 44 (9): 1337–1340. doi:10.1021/jm0108505. PMID 11311055.
- Zetler G (1988). "Neuroleptic-like, anticonvulsant and antinociceptive effects of aporphine alkaloids: bulbocapnine, corytuberine, boldine and glaucine". Archives Internationales De Pharmacodynamie Et De Therapie. 296: 255–281. PMID 2907279.
- Baldessarini RJ, Campbell A, Ben-Jonathan N, Ellingboe J, Zong R, Neumeyer JL (August 1994). "Effects of aporphine isomers on rat prolactin". Neuroscience Letters. 176 (2): 269–271. doi:10.1016/0304-3940(94)90098-1. PMID 7830962. S2CID 38264784.
- Bertol E, Fineschi V, Karch SB, Mari F, Riezzo I (February 2004). "Nymphaea cults in ancient Egypt and the New World: a lesson in empirical pharmacology". Journal of the Royal Society of Medicine. 97 (2): 84–85. doi:10.1177/014107680409700214. PMC 1079300. PMID 14749409.
- Seligman, Sian (2023-01-13). "Blue Lotus Flower: Smoking, Tea & More". DoubleBlind Mag. Retrieved 2023-01-19.
- Schimpf M, Ulmer T, Hiller H, Barbuto AF (August 2021). "Toxicity From Blue Lotus (Nymphaea caerulea) After Ingestion or Inhalation: A Case Series". Military Medicine. doi:10.1093/milmed/usab328. PMID 34345890.
- Grünblatt E, Mandel S, Berkuzki T, Youdim MB (July 1999). "Apomorphine protects against MPTP-induced neurotoxicity in mice". Movement Disorders. 14 (4): 612–618. doi:10.1002/1531-8257(199907)14:4<612::aid-mds1010>3.0.co;2-6. PMID 10435498.
- Scott KA, Qureshi MH, Cox PB, Marshall CM, Bellaire BC, Wilcox M, et al. (December 2020). "A Structural Analysis of the FDA Green Book-Approved Veterinary Drugs and Roles in Human Medicine". Journal of Medicinal Chemistry. 63 (24): 15449–15482. doi:10.1021/acs.jmedchem.0c01502. PMID 33125236. S2CID 226218045.
- Picada JN, Flores DG, Zettler CG, Marroni NP, Roesler R, Henriques JA (May 2003). "DNA damage in brain cells of mice treated with an oxidized form of apomorphine". Brain Research. Molecular Brain Research. 114 (1): 80–85. doi:10.1016/s0169-328x(03)00127-x. PMID 12782396.