Arachnid locomotion
Arachnid locomotion is the various means by which arachnids walk, run, or jump; they make use of more than muscle contraction, employing additional methods like hydraulic compression.[1][2] Another adaptation seen especially in larger arachnid variants is inclusion of elastic connective tissues.
Hydraulics
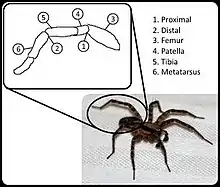
In most arachnids, hydraulic compression acts as the primary means of extension in several of their hinged leg joints, namely the femur–patella joint and tibia–metatarsus joints or second and third leg joints respectively.[3] Instead of blood, hemolymph is used to move nutrients around inside of the arachnid, and has the secondary function of acting as a hydraulic fluid. When compressed by the body of the arachnid, the hemolymph applies compressive force through channels in the limbs that cause them to extend.[4] This motion is then balanced by flexor muscle to retract the leg joints as needed. Due to hydraulics being used for extension, the flexor muscle is able to be significantly larger than would otherwise be possible without impacting size or weight.[5] Measurable core body volume change can occur during periods of higher compression to the legs, as the sinuses of the body contract to achieve pressurization in specific legs.[6] Aside from the normal gait of the arachnid, in some variants, extremely high pressures are used as a means of jumping, propelling rear legs and allowing for much greater and more sudden motion.[6]
Elastics
In larger variants of arachnids, such as the tarantulas and hairy desert spiders, another mechanism used for locomotion is an elastic sclerite.[6] These sclerites are semi-rigid connectors between leg segments that allow storage and expending of potential energy. This is used as a supplement or in conjunction with the hydraulics normally employed in those joints, allowing for greater weights to be carried, more rapid and sudden movement when combined with the already pronounced flexor muscle acting in those joints, as well as fine motor control with reduced sudden disruption of hemolymph flow.[6] At higher compression of the joint the stiffness of the sclerite has been found to increase significantly, denoting support even outside of normal tension.[6]
Influence on biomimetic design
Hydraulic locomotion in arachnids has acted as an inspiration for many modern biomimetic concepts in robotics intended for use by or with people, especially in the field of soft robotics. The use of hydraulics in robotic joints is aimed at replacing the more control heavy nature of modern robotics with a more passive system developed in soft actuation.[7] Various forms of actuation and force transmission can be achieved through these inspired designs, including rotation, lifting, and even damping effects.[8] The passive nature of the hydraulic and elastic extensor mechanisms employed have found use in orthotics projects aimed at assisting joints weakened by age or disease.[8]
Fluid secretion
An additional method used by some arachnids to improve locomotion is to secrete fluids, characterized by a hydrophobic effect, through the pads on the ends of their legs that are in contact with the walking surface.[9] It has been shown that the arachnid is capable of using the adhesive fluid selectively, meaning it can choose to not secrete the fluid in certain circumstances where it would be unwarranted such as in moist conditions.[9] The use of fluids allow the arachnid better traction through improved shear force for both standard locomotion and also sudden movements such as in jumping and leaping.
Challenges in modelling
Modelling the hydraulic system used by arachnids has been a challenge in the past due to scale and complexity. Simplified models focusing on individual joints and flow channels using modern imaging such as Micro-CT has allowed for mathematical expressions of pressure and flow acting on the joints.[3] Visualizing the flow of hemolymph in small bodies directly has been difficult due to resolution constraints and lack of contrast causing fluid and soft tissue being indistinguishable, but techniques have been employed using a combination of injected microbubbles as tracers in the hemolymph and synchrotron x-ray contrast imaging to track them.[10]
See also
References
- Bowerman, Robert F. (1981). "Arachnid Locomotion". In Herreid, Clyde F.; Fourtner, Charles R. (eds.). Locomotion and Energetics in Arthropods. Springer US. pp. 73–102. doi:10.1007/978-1-4684-4064-5_4. ISBN 978-1-4684-4066-9. Retrieved 2021-04-22.
- Spagna, Joseph C.; Peattie, Anne M. (2012-05-01). "Terrestrial locomotion in arachnids". Journal of Insect Physiology. 58 (5): 599–606. doi:10.1016/j.jinsphys.2012.01.019. PMID 22326455. Retrieved 2021-04-22.
- Liu, Chunbao; Chen, Shanshi; Sheng, Chuang; Ding, Peng; Qian, Zhihui; Ren, Lei (April 2019). "The art of a hydraulic joint in a spider's leg: modelling, computational fluid dynamics (CFD) simulation, and bio-inspired design". Journal of Comparative Physiology A. 205 (4): 491–504. doi:10.1007/s00359-019-01336-2. PMID 31032530. S2CID 135474232. Retrieved 1 March 2021.
- Weihmann, Tom; Gunther, Michael; Blickhan, Reinhard (October 2011). "Hydraulic leg extension is not necessarily the main drive in large spiders" (PDF). The Journal of Experimental Biology. 215 (4): 578–583. doi:10.1242/jeb.054585. PMID 22279064. Retrieved 1 March 2021.
- Hao, Xin; Ma, Wenxing; Liu, Chunbao; Li, Yilei; Qian, Zhihui; Ren, Luquan; Ren, Lei (December 2019). "Analysis of Spiders' Joint Kinematics and Driving Modes under Different Ground Conditions". Applied Bionics and Biomechanics. 2019: 1–9. doi:10.1155/2019/4617212. PMC 6935789. PMID 31929827.
- Sensenig, Andrew T.; Shultz, Jeffrey W. (November 2002). "Mechanics of cuticular elastic energy storage in leg joints lacking extensormuscles in arachnids" (PDF). The Journal of Experimental Biology. 206 (4): 771–784. doi:10.1242/jeb.00182. PMID 12517993. Retrieved 1 March 2021.
- Landkammer, Stefan; Winter, Florian; Schneider, Daniel; Hornfeck, Rudiger (July 2016). "Biomimetic Spider Leg Joints: A Review from Biomechanical Research to Compliant Robotic Actuators". Robotics. Special (3): 15. doi:10.3390/robotics5030015.
- Gaiser, I.; Wiegand, Roland; Ivlev, O.; Andres, A.; Breitwieser, H.; Schulz, S.; Bretthauer, G. (2012). "Compliant Robotics and Automation with Flexible Fluidic Actuators and Inflatable Structures" (PDF). Smart Actuation and Sensing Systems - Recent Advances and Future Challenges. doi:10.5772/51866. ISBN 978-953-51-0798-9. S2CID 53868418. Archived from the original (PDF) on 2019-03-03. Retrieved 1 March 2021.
- Peattie, Anne M.; Dirks, Jan-Henning; Henriques, Sergio; Federle, Walter (May 2011). "Arachnids Secrete a Fluid over Their Adhesive Pads". PLOS ONE. 6 (5): e20485. Bibcode:2011PLoSO...620485P. doi:10.1371/journal.pone.0020485. PMC 3102731. PMID 21637774.
- Lee, Wah-Keat; Socha, John J. (March 2009). "Direct visualization of hemolymph flow in the heart of a grasshopper (Schistocerca americana)". BMC Physiology. 2009: 2. doi:10.1186/1472-6793-9-2. PMC 2672055. PMID 19272159.