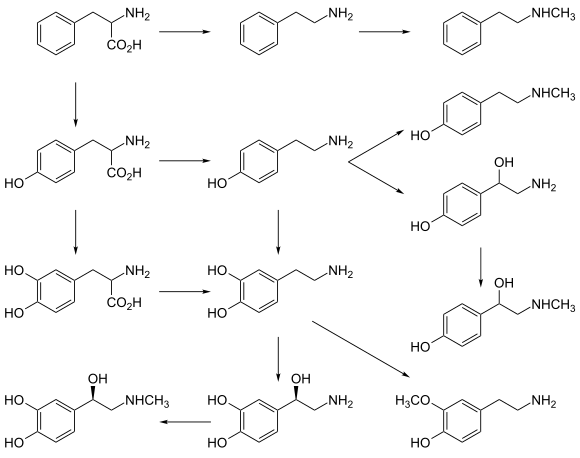
Biopterin-dependent aromatic amino acid hydroxylase
Biopterin-dependent aromatic amino acid hydroxylases (AAAH) are a family of aromatic amino acid hydroxylase enzymes which includes phenylalanine 4-hydroxylase (EC 1.14.16.1), tyrosine 3-hydroxylase (EC 1.14.16.2), and tryptophan 5-hydroxylase (EC 1.14.16.4). These enzymes primarily hydroxylate the amino acids L-phenylalanine, L-tyrosine, and L-tryptophan, respectively.
| Biopterin_H | |||||||||
|---|---|---|---|---|---|---|---|---|---|
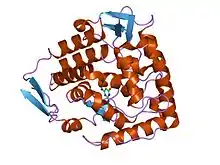 crystal structure of ternary complex of the catalytic domain of human phenylalanine hydroxylase (Fe(II)) complexed with tetrahydrobiopterin and norleucine | |||||||||
| Identifiers | |||||||||
| Symbol | Biopterin_H | ||||||||
| Pfam | PF00351 | ||||||||
| InterPro | IPR019774 | ||||||||
| PROSITE | PDOC00316 | ||||||||
| SCOP2 | 1toh / SCOPe / SUPFAM | ||||||||
| CDD | cd00361 | ||||||||
| |||||||||
The AAAH enzymes are functionally and structurally related proteins which act as rate-limiting catalysts for important metabolic pathways.[1] Each AAAH enzyme contains iron and catalyzes the ring hydroxylation of aromatic amino acids using tetrahydrobiopterin (BH4) as a substrate. The AAAH enzymes are regulated by phosphorylation at serines in their N-termini.
Role in metabolism
In humans, phenylalanine hydroxylase deficiency can cause phenylketonuria, the most common inborn error of amino acid metabolism.[2] Phenylalanine hydroxylase catalyzes the conversion of L-phenylalanine to L-tyrosine. Tyrosine hydroxylase catalyzes the rate-limiting step in catecholamine biosynthesis: the conversion of L-tyrosine to L-DOPA. Similarly, tryptophan hydroxylase catalyzes the rate-limiting step in serotonin biosynthesis: the conversion of L-tryptophan to 5-hydroxy-L-tryptophan.
Structure
It has been suggested that the AAAH enzymes each contain a conserved C-terminal catalytic (C) domain and an unrelated N-terminal regulatory (R) domain. It is possible that the R protein domains arose from genes that were recruited from different sources to combine with the common gene for the catalytic core. Thus, by combining with the same C domain, the proteins acquired the unique regulatory properties of the separate R domains.
References
- Grenett HE, Ledley FD, Reed LL, Woo SL (August 1987). "Full-length cDNA for rabbit tryptophan hydroxylase: functional domains and evolution of aromatic amino acid hydroxylases". Proc. Natl. Acad. Sci. U.S.A. 84 (16): 5530–4. Bibcode:1987PNAS...84.5530G. doi:10.1073/pnas.84.16.5530. PMC 298896. PMID 3475690.
- Erlandsen H, Fusetti F, Martinez A, Hough E, Flatmark T, Stevens RC (December 1997). "Crystal structure of the catalytic domain of human phenylalanine hydroxylase reveals the structural basis for phenylketonuria". Nat. Struct. Biol. 4 (12): 995–1000. doi:10.1038/nsb1297-995. PMID 9406548. S2CID 6293946.
- Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.