Arsinic acid
Arsinic acids are organoarsenic compounds with the formula R2AsO2H. They are formally, but not actually, related to arsinic acid, a hypothetical compound of the formula H2AsO2H. Arsinic acids are monoprotic, weak acids. They react with sodium sulfide to give the dithioarinates R2AsS2Na. [2] Arsinic acids are related to phosphinic acids (R2PO2H.).
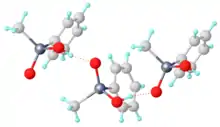
Structure of methylphenylarsinic acid showing intermolecular hydrogen-bonding.[1]
Well known arsinic acids include diphenylarsinic acid and cacodylic acid, R2AsO2H (R = Ph, Me, respectively).[3]
References
- Chan, Eric J.; Edmonds John S.; Kazawa Kozo; Skelton, Brian W.; White, Allan H. (2007). "The Crystal Structure of Methylphenylarsinic Acid: A Contaminant of Rice Plants and Groundwater". Chemistry Letters. 36: 160–161. doi:10.1246/cl.2007.160.
- Luminita Silaghi Dumitrescu; Ionel Haiduc; Johannes Weiss (1984). "Preparation and Properties of Some Organotin Dimethyl- and Diphenyldithioarsinates. The Crystal Structure of (CH3)2Sn[S2As(CH3)2]2". Journal of Organometallic Chemistry. 263 (2): 159–65. doi:10.1016/S0022-328X(00)99179-4.
- Henry B.F.Dixon (1996). "The Biochemical Action of Arsonic Acids Especially As Phosphate Analogues". Advances in Inorganic Chemistry. Advances in Inorganic Chemistry. Vol. 44. pp. 191–227. doi:10.1016/S0898-8838(08)60131-2. ISBN 978-0-12-023644-2.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.