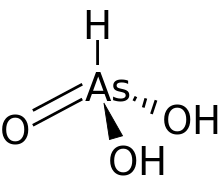
Arsonic acid
Arsonic acid is the simplest of the arsonic acids. It is a hypothetical compound, although the tautomeric arsenious acid (As(OH)3) is well established. In contrast to the instability of HAsO(OH)2, the phosphorus compound with analogous stoichiometry exists as the tetrahedral tautomer. Similarly, organic derivatives such as phenylarsonic acid are tetrahedral with pentavalent central atom.[3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Arsonic acid[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| AsH3O3 | |
| Molar mass | 125.943 g·mol−1 |
| Conjugate base | Hydrogen arsonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
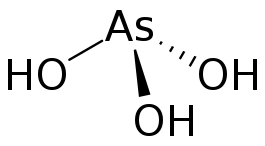
Arsenous acid (As(OH)3) is the stable tautomer of H3AsO3.[2]
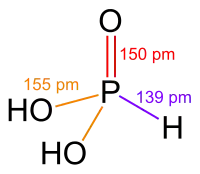
Phosphorous acid (also called phosphonic acid) exists as the pentavalent tautomer, in contrast to H3AsO3.
There are similar acids that are the same except for having different pnictogens. The phosphorus equivalent is phosphonic acid.
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 916. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- Munoz-Hernandez, M.-A. (1994). "Arsenic: Inorganic Chemistry". In King, R. B. (ed.). Encyclopedia of Inorganic Chemistry. Chichester: John Wiley & Sons.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.