Aureolysin
Aureolysin (EC 3.4.24.29, protease III, staphylococcal metalloprotease, Staphylococcus aureus neutral proteinase) is an extracellular metalloprotease expressed by Staphylococcus aureus.[1][2][3][4][5] This protease is a major contributor to the bacterium's virulence, or ability to cause disease, by cleaving host factors of the innate immune system as well as regulating S. aureus secreted toxins and cell wall proteins.[6][7] To catalyze its enzymatic activities, aureolysin requires zinc and calcium which it obtains from the extracellular environment within the host.[6][7]
Genetics
Aureolysin is expressed from the gene aur, which is located on a monocistronic operon.[8] The gene exists in two allelic forms but the sequence is highly conserved with 89% homology between the two.[9] The gene contains a coding sequence of 1,527 nucleotides that translates into a pre-pro-form of the enzyme that is 509 amino acids long.[9] Of the 509 amino acids, only 301 denote the mature form of aureolysin.[9] After translation, the pre-portion of the enzyme is a 27 amino acid N-terminal signal peptide that acts as a guide to the secretion system located within the cell wall.[9] Here, the signal peptide is cleaved upon secretion of aureolysin.[9]
Aureolysin is largely co-expressed with other major proteases of S. aureus including the two cystine proteases, Staphopain A (ScpA) and B (SspB), and a serine protease V8 (SspA). The transcriptional regulation of aur is controlled by "housekeeping" sigma factor σA, and is up-regulated by accessory gene regulator agr. Expression levels of aureolysin are at their highest during the post-exponential phase however, up-regulation of aureolysin during phagocytosis has also been observed.[10] Transcription is repressed by staphylococcal accessory regulator sarA and by alternative sigma factor σB (a stress response modulator of Gram-positive bacteria).
The aur gene has a high prevalence in the genome of both commensal- and pathogenic-type S. aureus strains.[11]
Activation
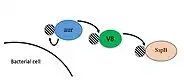
Aureolysin, along with V8, SspB, and ScpA, are all secreted a zymogens. This means that they are secreted in an inactive conformation until the propeptide is removed in some manner. Aureolysin, V8, and SspB constitute what is known as the staphylococcal proteolytic cascade.[8] All three of these proteases are secreted into the environment with the propeptide inhibiting their activation. Aureolysin undergoes autocatalysis and the propeptide is degraded generating the mature form of the enzyme.[8] Mature aureolysin will then cleave the propeptide from V8, causing this protease to become active.[8] Finally V8 will cleave SspB propeptide and the cascade is now complete.[8] ScpA becomes mature by autocatalytic degradation of the propeptide, similar to that of aureolysin.[8]
The active residues of aureolysin are of critical importance to its enzymatic function.[9] The active residue is a glutamate amino acid located at the 145th position of the protein.[9]
Immune Evasion
Aureolysin cleaves various immune components and host proteins. It is important for hiding the bacterium from the immune system and is responsible for mediating the transition of a biofilm forming phenotype to a mobile and invasive one. There are many different targets of aureolysin and the effect on each is critical for the bacterium's virulence.
One major way aureolysin contributes to infection, is by inactivating certain targets within the complement system. Of all the proteases, aureolysin is the most effective against the complement cascade.[12] In all three pathways of complement activation, there is a target for the protease to manipulate. In the classical pathway, aureolysin not only decreases deposition of C1q on the S. aureus bacterial surface, it induces C1q to bind surfaces and deposit on commensal bacteria surfaces that typically do not activate the innate immune system.[12] Aureolysin has also been noted to produce high levels of C5a in human plasma, which leads to overstimulation of neutrophils that ultimately results in neutrophil death.[12] C3 is another major target of aureolysin. The active site has a high affinity for C3 and will cleave it into C3a and C3b however, the protein is cleaved two amino acid residues away from the native site that is recognized by the host C3 convertase.[7][12] The aureolysin derived C3a and C3b are further degraded by host complement inhibitor factor H and I.[7][12] In the lectin pathway, aureolysin inhibits MBL and ficolin binding which, in turn, reduce C3b deposition.[6]
Further immune evasion outside of the complement system occurs in various ways. Aureolysin cleaves and inactivates protease inhibitor α1-antichymotrypsin and partially inactivates α1-antitrypsin.[13] The cleavage of α1-antitrypsin generates a fragment chemotactic to neutrophils, and the cleavage of both protease inhibitors causes deregulation of neutrophil-derived proteolytic activity.[13] Aureolysin has also been shown to cleave the antimicrobial peptide LL-37, rendering it inactive and unable to puncture the bacterial cell wall. Production of immunoglobulin by lymphocytes is inhibited by aureolysin as well.[5] It contributes to both coagulation triggered by coagulase, and to fibrinolysis mediated by staphylokinase.[13] Proteolytic conversion of pro-thrombin into thrombin by aureolysin works synergistically with coagulase and contributes to the staphylocoagulation of human plasma.[13] By inducing staphylocoagulation, the bacterium is hidden within the clot from phagocytic cells. Contradictory to staphylocoagulation, aureolysin is responsible for the activation of urokinase, and inactivation of α2-antiplasmin and plasminogen activator inhibitor-1.[7] This promotes the dissemination of the bacterium to allow for further invasion of the host.
Biological significance
When S. aureus is establishing an infection within a host, it needs to continuously switch from a static, or biofilm forming phenotype, to an invasive, or mobile phenotype. The proteases help mediate this process. Aureolysin appears to down-regulate the formation of biofilms and allow for the mobility of the bacterium. One way it contributes to this change is by mediating coagulation as well as the activation of urokinase. However, it also mediates S. aureus cell wall and secreted proteins to promote this change. For example, clumping factor B is a surface protein that is responsible for the binding of fibrinogen around the bacterium to hide it within a clot.[5][11] Aureolysin is responsible for the cleavage of clumping factor B, which causes the loss of S. aureus binding to fibrinogen. By this mechanism, it may act as a self-regulatory mechanism for dissemination and spreading in combination with activation of fibrinolysis, while the protease simultaneously provides protection against complement activation.[5][11] It has been demonstrated that aureolysin has impact for bacterial survival in human whole blood.[14] Aureolysin is also up-regulated upon phagocytosis and promotes intracellular survival.[5][10][15]
S. aureus prefers to establish a chronic, or long lasting infection within a host. While promoting dissemination and counteracting immune mechanisms, aureolysin also regulates secreted virulence factors to control the pathogenicity of the bacterium. By inactivation of PSMs and α-toxins, aureolysin may suppress the pathogenic impact of the bacteria allowing for a chronic infection to be established.[5]
References
- Arvidson, S. (1973). "Studies on extracellular proteolytic enzymes from Staphylococcus aureus. II. Isolation and characterization of an EDTA-sensitive protease". Biochim. Biophys. Acta. 302 (1): 149–157. doi:10.1016/0005-2744(73)90017-x. PMID 4632563.
- Saheb SA (1976). "Purification and characterization of an extracellular protease from Staphylococcus aureus inhibited by EDTA". Biochimie (in French). 58 (7): 793–804. doi:10.1016/s0300-9084(76)80310-0. PMID 823980.
- Drapeau GR (November 1978). "Role of metalloprotease in activation of the precursor of staphylococcal protease". Journal of Bacteriology. 136 (2): 607–13. doi:10.1128/JB.136.2.607-613.1978. PMC 218585. PMID 711676.
- Potempa J, Porwit-Bobr Z, Travis J (December 1989). "Stabilization vs. degradation of Staphylococcus aureus metalloproteinase". Biochimica et Biophysica Acta (BBA) - General Subjects. 993 (2–3): 301–4. doi:10.1016/0304-4165(89)90181-5. PMID 2512988.
- Potempa J, Shaw LN (2013-01-01). Rawlings ND, Salvesen G (eds.). Handbook of Proteolytic Enzymes. Academic Press. pp. 563–569. doi:10.1016/b978-0-12-382219-2.00114-9. ISBN 9780123822192.
- Laarman, Alexander J.; et al. (April 2011). "Staphylococcus aureus Metalloprotease Aureolysin Cleaves Complement C3 To Mediate Immune Evasion". Journal of Immunology. 186 (11): 6445–6453. doi:10.4049/jimmunol.1002948. PMID 21502375.
- Pietrocola, Giampiero; et al. (30 August 2017). "Staphylococcus aureus Manipulates Innate Immunitiy through Own and Host-Expressed Proteases". Frontiers in Cellular and Infection Microbiology. 7: 166. doi:10.3389/fcimb.2017.00166. PMC 5418230. PMID 28529927.
- Shaw L, Golonka E, Potempa J, Foster SJ (January 2004). "The role and regulation of the extracellular proteases of Staphylococcus aureus". Microbiology. 150 (Pt 1): 217–28. doi:10.1099/mic.0.26634-0. PMID 14702415.
- Sabat, A; et al. (February 2000). "Two Allelic Forms of Aureolysin Gene (aur) within Staphylococcus aureus". Infection and Immunity. 68 (2): 973–976. doi:10.1128/IAI.68.2.973-976.2000. PMC 97234. PMID 10639475.
- Burlak C, Hammer CH, Robinson MA, Whitney AR, McGavin MJ, Kreiswirth BN, Deleo FR (May 2007). "Global analysis of community-associated methicillin-resistant Staphylococcus aureus exoproteins reveals molecules produced in vitro and during infection". Cellular Microbiology. 9 (5): 1172–90. doi:10.1111/j.1462-5822.2006.00858.x. PMC 2064037. PMID 17217429.
- Dubin G (2002-07-01). "Extracellular proteases of Staphylococcus spp". Biological Chemistry. 383 (7–8): 1075–86. doi:10.1515/BC.2002.116. PMID 12437090. S2CID 23295763.
- Jusko, Monika; Potempa, Jan; Kantyka, Tomasz; Bielecka, Ewa; Miller, Halie K.; Kalinska, Magdalena; Dubin, Grzegorz; Garred, Peter; Shaw, Lindsey N. (2014). "Staphylococcal Proteases Aid in Evasion of the Human Complement System". Journal of Innate Immunity. 6 (1): 31–46. doi:10.1159/000351458. ISSN 1662-8128. PMC 3972074. PMID 23838186.
- Dubin, Grzegorz (July 2002). "Extracellular proteases of Staphylococcus spp". Biological Chemistry. 383 (7–8): 1075–1086. doi:10.1515/BC.2002.116. ISSN 1431-6730. PMID 12437090. S2CID 23295763.
- Jusko M, Potempa J, Kantyka T, Bielecka E, Miller HK, Kalinska M, Dubin G, Garred P, Shaw LN, Blom AM (2014-01-01). "Staphylococcal proteases aid in evasion of the human complement system". Journal of Innate Immunity. 6 (1): 31–46. doi:10.1159/000351458. PMC 3972074. PMID 23838186.
- Kubica M, Guzik K, Koziel J, Zarebski M, Richter W, Gajkowska B, Golda A, Maciag-Gudowska A, Brix K, Shaw L, Foster T, Potempa J (January 2008). "A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages". PLOS ONE. 3 (1): e1409. Bibcode:2008PLoSO...3.1409K. doi:10.1371/journal.pone.0001409. PMC 2169301. PMID 18183290.
External links
- Aureolysin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Aureolysin at Universal Protein Resource (UniProt)