Azo violet
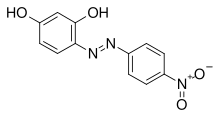
Azo violet (Magneson I;[1] p-nitrobenzeneazoresorcinol) is an azo compound with the chemical formula C12H9N3O4. It is used commercially as a violet dye and experimentally as a pH indicator, appearing yellow below pH 11, and violet above pH 13.[2] It also turns deep blue in the presence of magnesium salt in a slightly alkaline, or basic, environment.[3][4] Azo violet may also be used to test for the presence of ammonium ions. The color of ammonium chloride or ammonium hydroxide solution will vary depending upon the concentration of azo violet used. Magneson I is used to test Be also; it produces an orange-red lake with Be(II) in alkaline medium.[5]
 | |
| Names | |
|---|---|
| IUPAC name
4-[(E)-(4-Nitrophenyl)diazenyl]benzene-1,3-diol | |
| Other names
(E)-4-[(4-Nitrophenyl)diazenyl]benzene-1,3-diol 4-(4-Nitrophenyl)azobenzene-1,3-diol Magneson I p-Nitrophenylazoresorcinol 4-Nitrophenylazoresorcinol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.735 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H9N3O4 | |
| Molar mass | 259.318 g mol−1 |
| Appearance | dark red to brown crystalline powder |
| Density | 1.45 g/cm3 |
| 1 g/L H2O; 4 g/L Ethanol | |
| Hazards | |
| Flash point | 261.7 °C (503.1 °F; 534.8 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
| Azo violet (pH indicator) | ||
| below pH 11.0 | above pH 13.0 | |
| 11.0 | ⇌ | 13.0 |
Properties
.jpg.webp)
The intense color from which the compound gets its name results from irradiation and subsequent excitation and relaxation of the extended π electron system across the R-N=N-R' linked phenols. Absorption of these electrons falls in the visible region of the electromagnetic spectrum. Azo violet's intense indigo color (λmax 432 nm) approximates Pantone R: 102 G: 15 B: 240.
Synthesis
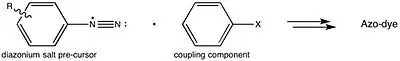
Azo violet can be synthesised by reacting 4-nitroaniline with nitrous acid (generated in situ with an acid and a nitrite salt) to produce a diazonium intermediate. This is then reacted with resorcinol, dissolved in a sodium hydroxide solution, via an azo coupling reaction.
This is consistent with the generalized strategy for preparing azo dyes.
Reactivity
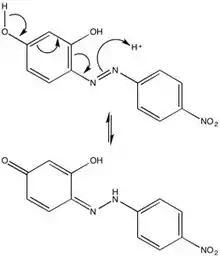
The chemical character of azo violet may be attributed to its azo group (-N=N-), six-membered rings, and hydroxyl side groups. Due to steric repulsions, azo violet is most stable in the trans-configuration, but isomerization of azo dyes by irradiation is not uncommon. The para-position tautomerization of azo violet provides mechanical insight into the behavior of the compound in an acidic environment, and thus its use as a basic pH indicator.
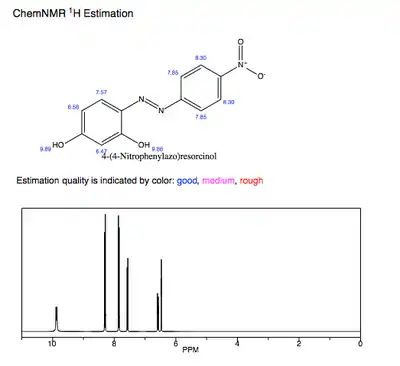
The predicted 1H-NMR of pure azo violet shows the hydroxyl protons as the most deshielded and acidic protons. The participation of these hydroxyl groups' electron-donation to the conjugated π system likewise influences azo violet's λmax and pKa value.
References
- "Magneson | C12H9N3O4 | ChemSpider". www.chemspider.com. Retrieved 2022-07-30.
- "Azo Violet 25GM from Cole-Parmer". Cole-Parmer. Archived from the original on 28 October 2016. Retrieved 28 October 2016.
- Feigl, F.; Anger, V. (2012-12-02). Spot Tests in Inorganic Analysis. Elsevier. ISBN 9780444597984.
- Gopalan, R. (2009-01-01). Inorganic Chemistry for Undergraduates. Universities Press. ISBN 9788173716607.
- Gopalan, R. (2009). Inorganic Chemistry for Undergraduates. Universities Press. ISBN 978-81-7371-660-7.