Balo concentric sclerosis
Baló's concentric sclerosis is a disease in which the white matter of the brain appears damaged in concentric layers, leaving the axis cylinder intact.[1] It was described by József Mátyás Baló who initially named it "leuko-encephalitis periaxialis concentrica" from the previous definition,[2] and it is currently considered one of the borderline forms of multiple sclerosis.
| Baló's concentric sclerosis | |
|---|---|
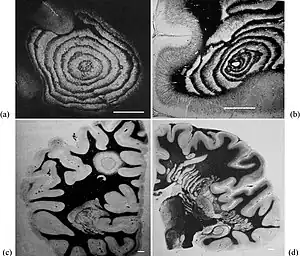 | |
| Typical aspects of Baló's concentric sclerosis. (a) Original case of Baló; several anastomoses are located in the lower half of the lesion. (b) Lesion centered by a veinule showing ring fragmentation in a constrained area. (c) Lesion. (d) Progress of the pathologic process from a center located in a constrained area, showing formation of bands. Loyez staining (myelin in black, destroyed areas in white); scale bars: 1 cm. | |
| Specialty | Neurology |
Baló's concentric sclerosis is a demyelinating disease similar to standard multiple sclerosis, but with the particularity that the demyelinated tissues form concentric layers. Scientists used to believe that the prognosis was similar to Marburg multiple sclerosis, but now they know that patients can survive, or even have spontaneous remission and asymptomatic cases.[3]
The concentric ring appearance is not specific to Baló's MS. Concentric lesions have also been reported in patients with neuromyelitis optica, standard MS, progressive multifocal leukoencephalopathy, cerebral autosomal dominant arteriopathy with subcortical infarcts, leukoencephalopathy, concomitant active hepatitis C and human herpes virus 6.[4]
Pathophysiology
The lesions of the Baló's sclerosis belong to the MS lesion pattern III (distal oligodendrogliopathy).[5] Balo concentric sclerosis is now believed to be a variant of pattern III multiple sclerosis[6] and probably due to metabolic problems.[7]
The Baló lesions show veins at their center, like those of MS, some suggestive of microhemorrhages or small ectatic venules. Unlike MS, no cortical gray matter lesions appear.[8]
Theoretical models
According with Dr. Lucchinetti investigations, in Baló's concentric sclerosis, the rings may be caused by a physiological hypoxia (similar to that caused by some toxins or viruses) in the lesion, which is in turn countered by expression of stress proteins at the border. This expression and counter-expression forms rings of preserved tissue within the lesion and rings of demyelinated tissue just beyond where the previous attack had induced the protective stress proteins. Hence, subsequent attacks form concentric rings.[9]
Some other researchers maintain that, as in pattern III MS, the mitochondrial respiratory chain complex IV activity is reduced and this could be the culprit of glutamate-mediated axonal injury.[10]
Ultimately, this expanding lesion causes the progressive picture typically seen. However, in some patients, the pathology underlying the disease appears to burn out and hence the disease may halt, hence the patients who spontaneously recover. The mechanisms triggering attacks and recovery remain uncertain.
Nevertheless, this model is questioned by recent reports that found astrocyte damage, similar to the one found in aquaporin-seropositive neuromyelitis optica. Though no anti-NMO antibodies have been found, the damage is similar, pointing to problems in the water channel of the astrocytes[11][12]
It presents three clinical subtypes: Monophasic, relapsing-remitting and primary rapidly progressive. Cerebrospinal fluid (CSF) is either normal or shows mild mononuclear inflammatory reaction. CSF-restricted oligoclonal bands are present only in a minority of cases[13][14]
Other models
A mathematical model for concentric sclerosis has been proposed.[15] Authors review the previous pathogenic theories, discuss the link between concentric sclerosis and Liesegang's periodic precipitation phenomenon and propose a new mechanism based on self-organization.
Clinical courses
The clinical course is primarily progressive, but a relapsing-remitting course has been reported.[16] It seems that the course gets better with prednisone therapy,[17] although evidence of this is anecdotal and such conclusions are difficult to accept given that there are cases where patients spontaneously recover whether the patient was on steroid therapy or not.
Baló lesions can disappear over time, but it has also been reported that the disease can convert to RRMS[18]
The clinical course of Balo-like lesions also depends to the context in which they appear. Balo-like lesions have been reported in aquaporin-4 seropositive and seronegative NMOSD, and also in children, as part of an ADEM-like presentation[19]
Diagnosis
Lesions under MRI are distinctive due to their natural concentric shape.
Under a lumbar puncture CSF test, with Baló's concentric sclerosis, as well as patients with pattern III lesions, were recently shown to be mostly negative for CSF-restricted oligoclonal bands.[13][20] Also pattern III patients tend to be negative under the MRZ-reaction (measles, rubeola and zoster viruses)[20]
Paediatric cases
Baló's concentric sclerosis in children has been reported to behave different from adults[21]
Lesions in autopsy and biopsy
A report comparing 1H-magnetic resonance spectroscopy, magnetization transfer and diffusion tensor imaging with histopathology in a patient with Baló's concentric sclerosis, found that inflammation was traced by fractional anisotropy and increased lactate. In contrast, magnetization transfer ratio and the diffusion coefficient show a loss of tissue in the rings of the lesion.[22]
Lesions under MRI
The features of the MRI and the characteristics of the lesion can be correlated when a biopsy has been taken, providing a way to standardize the future MRI diagnosis[23]
Baló's concentric sclerosis lesions can be distinguished from normal lesions on MRI showing alternating hypointense and hyperintense layers[24]
Baló's concentric lesions can be viewed using the myelin water imaging techniques. This is a special MRI sequence that shows the myelin's percentage of water content.[25]
Pattern III lesions, including Baló lesions, have a specific initiation pattern under MRI (MRILIP) consisting in showing Gadolinium enhancement before FLAIR MRI appearance.[26]
Under 7-Tesla MRI Ball lesions show a center vein, like in MS.[27]
Treatment
Treatment with corticosteroids is usual to relieve inflammation.
Epidemiology
Balo-like lesions have been reported to appear also in Tumefactive inflammatory leukoencephalopathy[28]
Associations
A possible association with psoriasis and autoimmune thyroiditis has been reported[29]
Pattern III (Baló-like) demyelinating spectrum
Baló-like lesions were classified as MS lesion pattern III in the MS spectrum. They have been reported alone, but also associated to standard multiple sclerosis, neuromyelitis optica, CADASIL and progressive multifocal leukoencephalopathy[30]
There is an overlap between what is considered Baló concentric sclerosis and some atypical cases of multiple sclerosis. A special subtype of multiple sclerosis presents Baló-like lesions (pattern III lesions) creating an intersection between these two conditions.[31]
Some patients with BCS present oligoclonal bands while others do not. It has been proposed that BCS lesions may not denote a single disease, but a final pathway of various demyelinating diseases, reflecting the presence of intralesional hypoxia as recently proposed[32]
Recently it has been reported that pattern III lesions are responsive to Mitoxantrone.[33] On the other hand, this pattern is the less responsive to plasmapheresis[34]
Pattern III lesions can be diagnosed without a biopsy because these patients show a high reactivity to AQP1 (without antibody) and varicella zoster virus (VZV).[35]
Origin of the lesions
Pattern III lesions were for sometime thought to be a MS nascent lesion, though it is not likely anymore.[36] A strain of bacterium Clostridium perfringens has been found in Pattern III lesions.[37] Tests in mice found that a toxin made by a rare strain of C. perfringens caused MS-like damage in the brain, and earlier work had identified this strain of C. perfringens in a human with MS.[38] MS patients were found to be 10 times more immune-reactive to the epsilon toxin than healthy people.[39]
Later reports state that Baló's patients showed loss of AQP1 and AQP4 in demyelinating lesions without binding auto-antibodies but with high reactivities to AQP1 peptides, which probably reflect astrocytic damage[35]
History
Though the disease carries the name of József Baló, it was first described by Otto Marburg in 1906.[40] Later, in 1928, József Baló studied the encephalitis periaxialis concentrica in a Hungarian patient, showing also demyelination of the peripheral nervous system.
References
- Baló J (1928). "Encephalitis periaxialis concentrica". Archives of Neurology and Psychiatry. 19 (2): 242–244. doi:10.1001/archneurpsyc.1928.02210080044002.
- Purohit; et al. (2015). "Balo's Concentric Sclerosis with Acute Presentation and Co-Existing Multiple Sclerosis-Typical Lesions on MRI". Case Reports in Neurology. 7 (1): 44–50. doi:10.1159/000380813. PMC 4386112. PMID 25873888.
- Karaarslan E, Altintas A, Senol U, et al. (August 2001). "Baló's concentric sclerosis: clinical and radiologic features of five cases". American Journal of Neuroradiology. 22 (7): 1362–1367. PMC 7975204. PMID 11498428.
- Ertuğrul Ö, Çiçekçi E, Cudi Tuncer M, Ufuk Aluçlu M (Oct 2018). "Balo's concentric sclerosis in a patient with spontaneous remission based on magnetic resonance imaging: A case report and review of literature". World J Clin Cases. 6 (11): 447–454. doi:10.12998/wjcc.v6.i11.447. PMC 6163147. PMID 30294609.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - (Article in Spanish) Archived 2007-04-30 at the Wayback Machine
- Popescu Bogdan F., Pirko Istvan, Lucchinetti Claudia F. (2013). "Pathology of Multiple Sclerosis: Where Do We Stand?". Continuum (Minneap Minn). 19 (4 Multiple Sclerosis): 901–921. doi:10.1212/01.CON.0000433291.23091.65. PMC 3915566. PMID 23917093.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Qiao Ling Cui, Malena Rone, Damla Khan, Melissa Bedard, Guillermina Almazan, Samuel Ludwin, Timophy Kennedy and Jack Antel, Oligodendrogliopathy in Multiple Sclerosis: Relation to Low Glycolytic Metabolic Rate of Oligodendrocytes (I10.004), Neurology 2016; vol. 86 no. 16 Supplement I10.004
- J. Behrens et al. 7 Tesla MRI of Balo's concentric sclerosis versus multiple sclerosis lesions, Annals of clinical and tranlational neurology, 29 June 2018, Volume5, Issue8, Pages 900-912, https://doi.org/10.1002/acn3.572
- Genetic susceptibility in MS – Steve Hauser. Rare Neuroimmunologic Disorders Symposium
- Mahad D. J., Ziabreva I., Campbell G., Lax N., White K., Hanson P. S., Lassmann H., Turnbull D. M. (2009). "Mitochondrial changes within axons in multiple sclerosis". Brain. 132 (5): 1161–1174. doi:10.1093/brain/awp046. PMC 3605917. PMID 19293237.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Matsuoka Takeshi; et al. (Nov 2010). "Aquaporin-4 astrocytopathy in Baló's disease". Acta Neuropathologica. 120 (5): 651–660. doi:10.1007/S00401-010-0733-7. PMID 20680636. S2CID 13604700.
- Kira J (Jul 2011). "Astrocytopathy in Balo's disease". Multiple Sclerosis. 17 (7): 771–779. doi:10.1177/1352458511400475. PMID 21459811. S2CID 24127230.
- Jarius S, Würthwein C, Behrens JR, Wanner J, Haas J, Paul F, Wildemann B (2018). "Baló's concentric sclerosis is immunologically distinct from multiple sclerosis: results from retrospective analysis of almost 150 lumbar punctures". Journal of Neuroinflammation. 15 (1): 22. doi:10.1186/s12974-017-1043-y. PMC 5774135. PMID 29347989.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Saeed Arif et al, Onion Peel Appearance in Balos Concentric Sclerosis, A Variant of Multiple Sclerosis, Journal of Ayub Medical College, Abbottabad, 2015;27(1)
- Khonsari RH, Calvez V (2007). Monk N (ed.). "The Origins of Concentric Demyelination: Self-Organization in the Human Brain". PLOS ONE. 2 (1): e150. Bibcode:2007PLoSO...2..150K. doi:10.1371/journal.pone.0000150. PMC 1764710. PMID 17225855.
- Moore GR, Berry K, Oger JJ, Prout AJ, Graeb DA, Nugent RA (December 2001). "Baló's concentric sclerosis: surviving normal myelin in a patient with a relapsing-remitting dinical course". Multiple Sclerosis. 7 (6): 375–382. doi:10.1177/135245850100700606. PMID 11795459. S2CID 10019226.
- Garbern J, Spence AM, Alvord EC (December 1986). "Balo's concentric demyelination diagnosed premortem". Neurology. 36 (12): 1610–1614. doi:10.1212/WNL.36.12.1610. PMID 3785678. S2CID 43010179.
- Novoselova OM, Il'Ves AG, Savintseva ZI, Prakhova LN, Zaplakhova OV, Bakhtiyarova KZ (2018). "[A case-report of Balo concentric sclerosis transformed into definite multiple sclerosis]". Zh Nevrol Psikhiatr Im S S Korsakova. 118 (8): 103–106. doi:10.17116/jnevro2018118082103. PMID 30160676.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Todd A. Hardy, Atypical Inflammatory Demyelinating Syndromes of the Central Nervous System, Neuroimmune Diseases pp 543-566, 14 August 2019
- Jarius S, König FB, Metz I, Ruprecht K, Paul F, Brück W, Wildemann B (2017). "Pattern II and pattern III MS are entities distinct from pattern I MS: evidence from cerebrospinal fluid analysis". Journal of Neuroinflammation. 14 (1): 171. doi:10.1186/s12974-017-0929-z. PMC 5576197. PMID 28851393.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Linnoila J, Chitnis T (2014). "Balo Concentric Sclerosis in Children: A Case Series". Journal of Child Neurology. 29 (5): 603–607. doi:10.1177/0883073813517294. PMID 24423690. S2CID 29304147.
- Lindquist S (2007). "Histopathology and serial, multimodal magnetic resonance imaging in a multiple sclerosis variant". Multiple Sclerosis. 13 (4): 471–482. doi:10.1177/1352458506071329. PMID 17463070. S2CID 35380552.
- Darke Bahador, Miller Litofsky, Ahsan (2013). "Baló's concentric sclerosis: imaging findings and pathological correlation". Journal of Radiology Case Reports. 7 (6): 1–8. doi:10.3941/jrcr.v7i6.1251. PMC 3888114. PMID 24421937.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Iannucci; et al. (2000). "Vanishing Balò-like lesions in multiple sclerosis". Journal of Neurology, Neurosurgery & Psychiatry. 69 (3): 399–400. doi:10.1136/jnnp.69.3.399. PMC 1737083. PMID 10945819.
- Laule Cornelia; et al. (2008). "Myelin water imaging of multiple sclerosis at 7 T: Correlations with histopathology". NeuroImage. 40 (4): 1575–1580. doi:10.1016/j.neuroimage.2007.12.008. PMID 18321730. S2CID 5855952.
- Charles; et al. (2015). "Multiple sclerosis lesion formation and early evolution revisited: A weekly high-resolution magnetic resonance imaging study". Multiple Sclerosis Journal. 22 (6): 761–769. doi:10.1177/1352458515600247. PMID 26362901. S2CID 3326176.
- Behrens JR; et al. (2018). "7 Tesla MRI of Balo's concentric sclerosis versus multiple sclerosis lesions". Annals of Clinical and Translational Neurology. 5 (8): 900–912. doi:10.1002/acn3.572. PMC 6093849. PMID 30128315.
- Lucas M.Pessini, Tumefactive inflammatory leukoencephalopathy in cocaine users: Report of three cases, Multiple Sclerosis and Related Disorders, Volume 38, February 2020, 101496
- Corina Roman-Filip, Aurelian Ungureanu, Ileana Praƒvariu, Baló-like Lesion With Psoriasis and Autoimmune Thyroiditis, Essays, UK. (November 2018)
- Commentary on Pique et al.’s paper entitled: Peripheral late reactivation of a previously typical monofocal Balo’s concentric sclerosis lesion
- Hardy Todd A, Tobin W Oliver, Lucchinetti Claudia F (2016). "Exploring the overlap between multiple sclerosis, tumefactive demyelination and Baló's concentric sclerosis". Multiple Sclerosis Journal. 22 (8): 986–992. doi:10.1177/1352458516641776. PMID 27037180. S2CID 3810418.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Jarius S, Würthwein C, Behrens JR, Wanner J, Haas J, Paul F, Wildemann B (2018). "Baló's concentric sclerosis is immunologically distinct from multiple sclerosis: results from retrospective analysis of almost 150 lumbar punctures". J Neuroinflammation. 15 (1): 22. doi:10.1186/s12974-017-1043-y. PMC 5774135. PMID 29347989.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Grüter Thomas, Metz Imke, Gahlen Anna, Kneiphof Janina, Stork Lidia, Brück Wolfgang, Gold Ralf, Kleiter Ingo (2018). "Mitoxantrone treatment in a patient with multiple sclerosis and pattern III lesions". Clinical and Experimental Neuroimmunology. 9 (3): 169–172. doi:10.1111/cen3.12466. S2CID 81454231.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Stork Lidia, Ellenberger David, Beißbarth Tim, Friede Tim, Lucchinetti Claudia F., Brück Wolfgang, Metz Imke (2018). "Differences in the Reponses to Apheresis Therapy of Patients With 3 Histopathologically Classified Immunopathological Patterns of Multiple Sclerosis". JAMA Neurology. 75 (4): 428–435. doi:10.1001/jamaneurol.2017.4842. PMC 5885209. PMID 29404583.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Stork; et al. (2020). "Antibody signatures in patients with histopathologically defined multiple sclerosis patterns". Acta Neuropathol. 139 (3): 547–564. doi:10.1007/s00401-019-02120-x. PMC 7035238. PMID 31950335.
- Wolfgang Brück MD Bogdan Popescu MD Claudia F. Lucchinetti MD Silva Markovic‐Plese MD, PhD Ralf Gold MD Dietmar Rudolf Thal MD Imke Metz MD, Neuromyelitis optica lesions may inform multiple sclerosis heterogeneity debate, 14 April 2012, https://doi.org/10.1002/ana.23621
- Rashid Rumah Kareem, Linden Jennifer, Fischetti Vincent A., Vartanian Timothy (2013). "Isolation of Clostridium perfringens Type B in an Individual at First Clinical Presentation of Multiple Sclerosis Provides Clues for Environmental Triggers of the Disease". PLOS ONE. 8 (10): e76359. Bibcode:2013PLoSO...876359R. doi:10.1371/journal.pone.0076359. PMC 3797790. PMID 24146858.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - "Multiple sclerosis 'linked to food bug'". BBC. 29 January 2014. Retrieved 29 January 2014.
- Woerner, Amanda (29 January 2014). "Bacterial toxin may trigger multiple sclerosis, research finds". Fox News.
- Commentary on Pique et al.’s paper entitled: Ciampi Ethel, Tur Carmen, Montalban Xavier (2015). "Peripheral late reactivation of a previously typical monofocal Baló's concentric sclerosis lesion". Multiple Sclerosis. 21 (8): 1084–1086. doi:10.1177/1352458515586090. PMID 26014610. S2CID 1020393.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Khonsari RH, Calvez V (September 2007). "Concentric demyelination by self-organization: a new hypothesis for Baló's sclerosis". Nature Clinical Practice Neurology. 3 (9): E1. doi:10.1038/ncpneuro0619. PMID 17805242. S2CID 33938721.