Chlamydia (genus)
Chlamydia is a genus of pathogenic Gram-negative bacteria that are obligate intracellular parasites. Chlamydia infections are the most common bacterial sexually transmitted diseases in humans and are the leading cause of infectious blindness worldwide.[1]
| Chlamydia | |
|---|---|
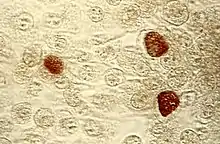 | |
| Chlamydia trachomatis inclusion bodies (brown) in a McCoy cell culture. | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Chlamydiota |
| Class: | Chlamydiia |
| Order: | Chlamydiales |
| Family: | Chlamydiaceae |
| Genus: | Chlamydia Jones et al. 1945 |
| Type species | |
| Chlamydia trachomatis (Busacca 1935) Rake 1957 | |
| Species | |
| |
| Synonyms | |
| |
Species include Chlamydia trachomatis (a human pathogen), Ch. suis (affects only swine), and Ch. muridarum (affects only mice and hamsters).[2] Humans mainly contract Ch. trachomatis, Ch. pneumoniae, Ch. abortus, and Ch. psittaci.[3]
Classification
Because of Chlamydia's unique developmental cycle, it was taxonomically classified in a separate order.[4] Chlamydia is part of the order Chlamydiales, family Chlamydiaceae.
In the early 1990s six species of Chlamydia were known. A major re-description of the Chlamydiales order in 1999, using the then new techniques of DNA analysis, split three of the species from the genus Chlamydia and reclassified them in the then newly created genus Chlamydophila, and also added three new species to this genus.[5] In 2001 many bacteriologists strongly objected to the reclassification,[6] although in 2006 some scientists still supported the distinctness of Chlamydophila.[7] In 2009 the validity of Chlamydophila was challenged by newer DNA analysis techniques, leading to a proposal to "reunite the Chlamydiaceae into a single genus, Chlamydia".[8] This appears to have been accepted by the community,[9][10] bringing the number of (valid) Chlamydia species up to 9. Many probable species were subsequently isolated, but no one bothered to name them. In 2013 a 10th species was added, Ch. ibidis, known only from feral sacred ibis in France.[11] Two more species were added in 2014 (but validated 2015): Ch. avium which infects pigeons and parrots, and Ch. gallinacea infecting chickens, guinea fowl and turkeys.[3] Ch. abortus was added in 2015, and the Chlamydophila species reclassified.[6] A number of new species were originally classified as aberrant strains of Ch. psittaci[3]
Genomes
Chlamydia species have genomes around 1.0 to 1.3 megabases in length.[12] Most encode ~900 to 1050 proteins.[13] Some species also contain a DNA plasmids or phage genomes (see Table). The elementary body contains an RNA polymerase responsible for the transcription of the DNA genome after entry into the host cell cytoplasm and the initiation of the growth cycle. Ribosomes and ribosomal subunits are found in these bodies.
| Ch. trachomatis MoPn | Ch. trachomatis D | Ch. pneumoniae AR39 | Ch. pneumoniae CWL029 | |
|---|---|---|---|---|
| Size (nt) | 1,069,412 | 1,042,519 | 1,229,853 | 1,230,230 |
| ORFs | 924 | 894 | 1052 | 1052 |
| tRNAs | 37 | 37 | 38 | 38 |
| plasmids | 1 (7,501 nt) | 1 (7,493 nt) | 1 ssDNA phage | none |
Table 1. Genome features of selected Chlamydia species and strains. MoPn is a mouse pathogen while strain "D" is a human pathogen. About 80% of the genes in Ch. trachomatis and Ch. pneumoniae are orthologs. Adapted after Read et al. 2000[13]
Developmental cycle
Chlamydia may be found in the form of an elementary body and a reticulate body. The elementary body is the nonreplicating infectious particle that is released when infected cells rupture. It is responsible for the bacteria's ability to spread from person to person and is analogous to a spore. The elementary body may be 0.25 to 0.30 μm in diameter. This form is covered by a rigid cell wall (hence the combining form chlamyd- in the genus name). The elementary body induces its own endocytosis upon exposure to target cells. One phagolysosome usually produces an estimated 100–1000 elementary bodies.
Chlamydia may also take the form of a reticulate body, which is in fact an intracytoplasmic form, highly involved in the process of replication and growth of these bacteria. The reticulate body is slightly larger than the elementary body and may reach up to 0.6 μm in diameter with a minimum of 0.5 μm. It does not have a cell wall. When stained with iodine, reticulate bodies appear as inclusions in the cell. The DNA genome, proteins, and ribosomes are retained in the reticulate body. This occurs as a result of the development cycle of the bacteria. The reticular body is basically the structure in which the chlamydial genome is transcribed into RNA, proteins are synthesized, and the DNA is replicated. The reticulate body divides by binary fission to form particles which, after synthesis of the outer cell wall, develop into new infectious elementary body progeny. The fusion lasts about three hours and the incubation period may be up to 21 days. After division, the reticulate body transforms back to the elementary form and is released by the cell by exocytosis.[4]
Studies on the growth cycle of Ch. trachomatis and Ch. psittaci in cell cultures in vitro reveal that the infectious elementary body (EB) develops into a noninfectious reticulate body (RB) within a cytoplasmic vacuole in the infected cell. After the elementary body enters the infected cell, an eclipse phase of 20 hours occurs while the infectious particle develops into a reticulate body. The yield of chlamydial elementary bodies is maximal 36 to 50 hours after infection.
A histone like protein HctA and HctB play role in controlling the differentiation between the two cell types. The expression of HctA is tightly regulated and repressed by small non-coding RNA, IhtA until the late RB to EB re-differentiation.[14] The IhtA RNA is conserved across Chlamydia species.[15]
Pathology
Most commonly, chlamydial infections[16] do not cause symptoms. However, for men, a burning sensation when urinating is often probable. For women, odor and itching are possible symptoms. Both sexes may notice more sebum production as the infection escalates, all which produces greasy sweat, more oily complexion, and can be misdiagnosed as acne eruptions rather than the whole body's hidden fight to defend itself from an STD. All people who have engaged in sexual activity with potentially infected individuals may be offered one of several tests to diagnose the condition.
Chlamydia can be detected through culture tests or nonculture tests. The main nonculture tests include fluorescent monoclonal antibody test, enzyme immunoassay, DNA probes, rapid Chlamydia tests and leukocyte esterase tests. Whereas the first test can detect the major outer membrane protein (MOMP), the second detects a colored product converted by an enzyme linked to an antibody. The rapid Chlamydia tests use antibodies against the MOMP, the leukocyte esterase tests detect enzymes produced by leukocytes containing the bacteria in urine.[4]
Evolution
Recent phylogenetic studies have revealed that Chlamydia likely shares a common ancestor with cyanobacteria, the group containing the endosymbiont ancestor to the chloroplasts of modern plants, hence, Chlamydia retains unusual plant-like traits, both genetically and physiologically. In particular, the enzyme L,L-diaminopimelate aminotransferase, which is related to lysine production in plants, is also linked with the construction of chlamydial peptidoglycan, which is required for division.[17] The genetic encoding for the enzymes is remarkably similar in plants, cyanobacteria, and Chlamydia, demonstrating a close common ancestry.[18]
Phylogeny
| 16S rRNA based LTP_01_2022[19][20][21] | 120 marker proteins based GTDB 07-RS207[22][23][24] | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
References
- Ryan KJ, Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 463–70. ISBN 978-0-8385-8529-0.
{{cite book}}:|author=has generic name (help) - Ward M. "Taxonomy diagram". Chlamydiae.com. Archived from the original on 2010-09-18. Retrieved 2008-10-28.
- Joseph, SJ; et al. (2015), "Chlamydiaceae genomics reveals interspecies admixture and the recent evolution of Chlamydia abortus infecting lower mammalian species and humans", Genome Biol Evol, 7 (11): 3070–3084, doi:10.1093/gbe/evv201, PMC 4994753, PMID 26507799.
- "Chlamydia trachomatis". Archived from the original on July 2, 2010. Retrieved June 18, 2010.
- Everett KD, Bush RM, Andersen AA (April 1999). "Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms". Int. J. Syst. Bacteriol. 49 (2): 415–40. doi:10.1099/00207713-49-2-415. PMID 10319462.
- Parte, A.C. "Chlamydia". LPSN.
- Griffiths E, Ventresca MS, Gupta RS (2006). "BLAST screening of chlamydial genomes to identify signature proteins that are unique for the Chlamydiales, Chlamydiaceae, Chlamydophila and Chlamydia groups of species". BMC Genomics. 7: 14. doi:10.1186/1471-2164-7-14. PMC 1403754. PMID 16436211.
- Stephens RS, Myers G, Eppinger M, Bavoil PM (March 2009). "Divergence without difference: phylogenetics and taxonomy of Chlamydia resolved". FEMS Immunol. Med. Microbiol. 55 (2): 115–9. doi:10.1111/j.1574-695X.2008.00516.x. PMID 19281563.
- Greub, Gilbert (1 November 2010). "International Committee on Systematics of Prokaryotes Subcommittee on the taxonomy of the Chlamydiae Minutes of the inaugural closed meeting, 21 March 2009, Little Rock, AR, USA". International Journal of Systematic and Evolutionary Microbiology. 60 (11): 2691–2693. doi:10.1099/ijs.0.028225-0. PMID 21048221.
- Balsamo, G; et al. (2017), "Compendium of measures to control Chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis), 2017" (PDF), J Avian Med Surg, 31 (3): 262–282, doi:10.1647/217-265, PMID 28891690, S2CID 26000244.
- Vorimore, Fabien; Hsia, Ru-ching; Huot-Creasy, Heather; Bastian, Suzanne; Deruyter, Lucie; Passet, Anne; Sachse, Konrad; Bavoil, Patrik; Myers, Garry; Laroucau, Karine (20 September 2013). "Isolation of a New Chlamydia species from the Feral Sacred Ibis (Threskiornis aethiopicus)- Chlamydia ibidis". PLOS ONE. 8 (9). e74823. Bibcode:2013PLoSO...874823V. doi:10.1371/journal.pone.0074823. PMC 3779242. PMID 24073223.
- "EMBL bacterial genomes". Retrieved January 19, 2012.
- Read, T. D.; Brunham, R. C.; Shen, C.; Gill, S. R.; Heidelberg, J. F.; White, O.; Hickey, E. K.; Peterson, J.; Utterback, T. (2000-03-15). "Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39". Nucleic Acids Research. 28 (6): 1397–1406. doi:10.1093/nar/28.6.1397. ISSN 1362-4962. PMC 111046. PMID 10684935.
- Grieshaber, NA; Grieshaber, SS; Fisher, ER; Hackstadt, T (2006). "A small RNA inhibits translation of the histone-like protein Hc1 in Chlamydia trachomatis". Mol. Microbiol. 59 (2): 541–50. doi:10.1111/j.1365-2958.2005.04949.x. PMID 16390448. S2CID 11872982.
- Tattersall, J; Rao, GV; Runac, J; Hackstadt, T; Grieshaber, SS; Grieshaber, NA (2012). "Translation inhibition of the developmental cycle protein HctA by the small RNA IhtA is conserved across Chlamydia". PLOS ONE. 7 (10): e47439. Bibcode:2012PLoSO...747439T. doi:10.1371/journal.pone.0047439. PMC 3469542. PMID 23071807.
- "Chlamydia protection". Retrieved August 1, 2010.
- Liechti, G. W.; Kuru, E.; Hall, E.; Kalinda, A.; Brun, Y. V.; VanNieuwenhze, M.; Maurelli, A. T. (February 2014). "A new metabolic cell-wall labelling method reveals peptidoglycan in Chlamydia trachomatis". Nature. 506 (7489): 507–510. doi:10.1038/nature12892. ISSN 1476-4687. PMC 3997218. PMID 24336210.
- McCoy AJ, Adams NE, Hudson AO, Gilvarg C, Leustek T, Maurelli AT (2006). "L,L-diaminopimelate aminotransferase, a trans-kingdom enzyme shared by Chlamydia and plants for synthesis of diaminopimelate/lysine". Proc. Natl. Acad. Sci. U.S.A. 103 (47): 17909–14. Bibcode:2006PNAS..10317909M. doi:10.1073/pnas.0608643103. PMC 1693846. PMID 17093042.
- "The LTP". Retrieved 23 February 2022.
- "LTP_all tree in newick format". Retrieved 23 February 2022.
- "LTP_01_2022 Release Notes" (PDF). Retrieved 23 February 2022.
- "GTDB release 07-RS207". Genome Taxonomy Database. Retrieved 20 June 2022.
- "bac120_r207.sp_labels". Genome Taxonomy Database. Retrieved 20 June 2022.
- "Taxon History". Genome Taxonomy Database. Retrieved 20 June 2022.
Further reading
 Data related to Chlamydia at Wikispecies
Data related to Chlamydia at Wikispecies- Chlamydiae.com