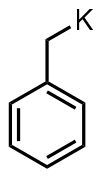
Benzyl potassium
Benzylpotassium is an organopotassium compound with the formula C6H5CH2K. It is an orange powder. Like organo-alkali metal reagents in general, benzyl potassium is highly reactive, so much so that it reacts with most solvents. It is highly air sensitive.
 | |
| Names | |
|---|---|
| Other names
Potassium benzyl | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H7K | |
| Molar mass | 130.231 g·mol−1 |
| Appearance | Orange solid |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Ignites in air |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
One early synthesis proceeds by two-step transmetallation reaction via p-tolylpotassium:[1]
- (CH3C6H4)2Hg + 2 K → 2 CH3C6H4K + Hg
- CH3C6H4K → KCH2C6H5
A modern synthesis involves the reaction of butyllithium, potassium tert-butoxide, and toluene.[2] Although potassium hydride can also be used as a strong base for preparing potassium salts, benzyl potassium has the advantage of being molecular and hence more fast-acting.
References
- Gilman, Henry; Pacevitz, Henry A; Baine, Ogden (1940). "Benzylalkali Compounds1". Journal of the American Chemical Society. 62 (6): 1514. doi:10.1021/ja01863a054.
- Lochmann, L; Trekoval, J (1987). "Lithium-potassium exchange in alkyllithium/potassium t-pentoxide systems". Journal of Organometallic Chemistry. 326: 1. doi:10.1016/0022-328X(87)80117-1.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.