Biological basis of personality
The biological basis of personality is the collection of brain systems and mechanisms that underlie human personality. Human neurobiology, especially as it relates to complex traits and behaviors, is not well understood, but research into the neuroanatomical and functional underpinnings of personality are an active field of research. Animal models of behavior, molecular biology, and brain imaging techniques have provided some insight into human personality, especially trait theories.
Much of the current understanding of personality from a neurobiological perspective places an emphasis on the biochemistry of the behavioral systems of reward, motivation, and punishment. This has led to a few biologically based personality theories such as Eysenck's three factor model of personality, Grey's reinforcement sensitivity theory (RST), and Cloninger's model of personality. The Big Five model of personality is not biologically based; yet some research in the differences in brain structures provided biological support also for this model.
Defining personality in a biological context
Personality can be defined as a set of characteristics or traits that drive individual differences in human behavior. From a biological perspective, these traits can be traced back to brain structures and neural mechanisms. However, this definition and theory of biological basis is not universally accepted. There are many conflicting theories of personality in the fields of psychology, psychiatry, philosophy, and neuroscience. A few examples of this are the nature vs. nurture debate and how the idea of a 'soul' fits into biological theories of personality.[1]
History of biology-based personality research

Since the time of the ancient Greeks, humankind has attempted to explain personality through spiritual beliefs, philosophy, and psychology. Historically, studies of personality have traditionally come from the social sciences and humanities, but in the past two decades neuroscience has begun to be more influential in the understanding of human personality.[2]
However, the most cited and influential figures in publishing the first biology-based personality theories are Hans Eysenck and Jeffrey Alan Gray. Eysenck used both behavioral and psychophysiological methodologies to test and develop his theories.[3] He published a book in 1947 called Dimensions of Personality, describing the personality dimensions of extraversion and neuroticism. Gray, a student of Eysenck, studied personality traits as individual differences in sensitivity to rewarding and punishing stimuli.[3] The significance of Gray's work and theories was his use of biology to define behavior, which stimulated a lot of subsequent research.[4]
In 1951, Hans Eysenck and Donald Prell published an experiment in which identical (monozygotic) and fraternal (dizygotic) twins, ages 11 and 12, were tested for neuroticism. It is described in detail in an article published in the Journal of Mental Science in which Eysenck and Prell concluded that "The factor of neuroticism is not a statistical artifact, but constitutes a biological unit which is inherited as a whole ... neurotic predisposition is to a large extent hereditarily determined."[5] The study concluded that the neuroticism trait was a result of up to eighty percent of genetics. There was a stronger correlation among identical twins rather than fraternal twins.[6]
The idea of biology-based personality research is relatively new, but growing in interest and number of publications.[7] In August 2004, there was a conference specifically on the topic, called The Biological Basis of Personality and Individual Differences.[8] This allowed for presenting and sharing of ideas between psychologists, psychiatrists, molecular geneticists, and neuroscientists, and eventually gave birth to the book under the same title.[8] The book is a collection of current research (as of 2006) in the field contributed by many authors and edited by Turhan Canli. Recently, psychology professor Colin G. DeYoung has even named the idea as the field of "Personality Neuroscience."[9] Furthermore, a journal devoted to cultivating research investigating the neurobiological basis of personality has recently been established and is called "Personality Neuroscience."[10]
Personality theories with biological basis
There are many theories of personality that centre on the identification of a set of traits that encompass human personality. Few however, are biologically based. This section will describe some theories of personality that have a biological basis.
Eysenck's three-factor model of personality
Eysenck's three-factor model of personality was a causal theory of personality based on activation of reticular formation and limbic system. The reticular formation is a region in the brainstem that is involved in mediating arousal and consciousness. The limbic system is involved in mediating emotion, behavior, motivation, and long-term memory.
- Extraversion (E) – degree to which people are outgoing and are interactive with people, which is mediated by the activation of the reticular formation.
- Neuroticism (N) – degree of emotional instability, which is associated with the limbic system.
- Psychoticism (P) – degree of aggression and interpersonal hostility.
Gray's reinforcement sensitivity theory
Gray's reinforcement sensitivity theory (RST) is based on the idea that there are three brain systems that all differently respond to rewarding and punishing stimuli.[3]
- Fight-flight-freeze system (FFFS) – mediates the emotion of fear (not anxiety) and active avoidance of dangerous situations. The personality traits associated with this system is fear-proneness and avoidance.
- Behavioral inhibition system (BIS) – mediates the emotion of anxiety and cautious risk-assessment behavior when entering dangerous situations due to conflicting goals. The personality traits associated with this system is worry-proneness and anxiety.
- Behavioral approach system (BAS) – mediates the emotion of 'anticipatory pleasure,' resulting from reactions to desirable stimuli. The personality traits associated with this system are optimism, reward-orientation, and impulsivity.
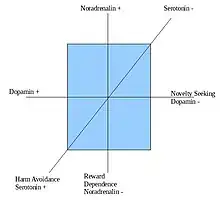
Cloninger model of personality
This model of personality is based on the idea that different responses to punishing, rewarding, and novel stimuli the main characteristics of the human mind is caused by an interaction of the three dimensions below:
- Novelty Seeking (NS) – degree to which people are impulsive, correlated with low dopamine activity.
- Harm Avoidance (HA) – degree to which people are anxious, correlated with high serotonin activity.
- Reward Dependence (RD) – degree to which people are approval seeking, correlated with low norepinephrine activity.
Five factor model of personality
The five factor model (also known as the Big Five) is a widely used personality assessment that describes five core traits that a person possesses:
- Openness – degree to which people enjoy experiencing new stimuli
- Conscientiousness – degree to which people are dutiful and goal-oriented
- Extraversion – degree to which people seek stimuli outside of themselves
- Agreeableness – degree to which people aim to cooperate and please others
- Neuroticism – degree to which people are emotionally unstable
There is large body of research relating the Big Five traits to individual differences in the brain's structure and function, as measured by MRI-based techniques. A selection of these findings are outlined in the "Brain imaging basis of personality" section below.
Two factor model of personality
A higher-order factor structure can be derived from the Big Five traits, as these traits have often been found to be correlated. Agreeableness, Conscientiousness, and Neuroticism (reversed) can be distilled into a single factor α, or the Stability factor. On the other hand, Extraversion and Openness can be distilled into a single factor β, or the Plasticity factor.[11][12] These two meta-traits have been shown to be significantly heritable using behavior genetic analysis,[13] which suggests a neurobiological basis that is unique and specific to these meta-traits. Indeed, a growing body of evidence demonstrates that serotonin is associated with Stability and dopamine is associated with Plasticity.[11][12][14]
Experimental techniques
There are many experimental techniques for measuring the biology of the brain, but there are five main methods used to investigate the biological basis of personality.[15] The biological data from these methods are commonly correlated with personality traits. These personality traits are often determined by personality questionnaires. However, personality questionnaires may be biased because they are self-reported. As a result, scientists emphasize using several different measures of personality,[15][16] rather than solely self-reported measures of personality. For example, another measure of personality traits is observation of behavior. Both humans and animals have been observed to measure personality traits, but animals are particularly useful for studying the long-term behavioral-biological relationship of personality.[17]
Another interesting method that has become more sophisticated and affordable to researchers is the method of whole genome expression analysis. This method involves collecting data for a large number of genes simultaneously which provides many advantages in studying personality. In an article written by Alison M. Bell and Nadia Aubin-Horth, they describe the advantages very clearly by stating, "For one, it is probable that the genetic basis of personality is polygenic, so it makes sense to simultaneously study many genes. In addition, gene products rarely act alone. Instead, they perform their function by interacting together in pathways and networks. As a result, the molecular changes that characterize a phenotype are frequently not based on a single marker or gene, but rather on an entire pathway. Whole genome expression profiling therefore has the potential to reveal new candidates genes and pathways."[18]
| Method | Function | Significance |
|---|---|---|
| Electroencephalography (EEG) | This method measures electrical activity on the surface of the brain through the scalp, and has the high temporal resolution.[15] | Before the advent of brain imaging technology, the only method to measure brain activity was electroencephalography (EEG).[15] |
| Brain Imaging | Brain imaging can refer to either structural or functional imaging. Structural imaging allows for analysis using structural characteristics of the brain, whereas functional imaging involves measuring brain activity. Structural imaging of the brain can be accomplished by using Magnetic Resonance Imaging (MRI). Examples of functional imaging methods include Positron Emission Tomography (PET) and functional MRI (fMRI). PET scans measure the metabolism associated with brain activity, and fMRI measures the flow of blood in the brain, which reflects local brain activity. MRI has particularly high spatial resolution and is entirely non-invasive, whereas PET scans require the injection of radioactive tracers. | Brain imaging has catalyzed research of the neurobiological correlates of personality.[3] |
| Molecular genetics | This method is used to analyze a gene-trait link, by measuring the structure and function of genes in the brain.[15] | The use of molecular genetics in biology-based personality research is expected to grow.[7] |
| Molecular assays | This method is used to analyze the amount of psychoactive substances, such as hormones and neurotransmitters. | Together, these two methods can specifically quantify, define, and manipulate the effects of brain molecules on behavior and personality traits. This has great clinical significance for treatment of personality disorders. |
| Pharmacological Manipulation | This method is used to alter the levels of biochemicals, and observe the effects on behavior. | |
Genetic and molecular correlations to personality
Neurotransmitters

The biology-based personality theories (discussed below) are based on correlating personality traits with behavioral systems related to motivation, reward, and punishment. On a broad level, this involves the autonomic nervous system, fear-processing circuits in the amygdala, the reward pathway from the ventral tegmental area (VTA) to the nucleus accumbens and prefrontal cortex. All of these circuits heavily rely on neurotransmitters and their precursors, but there has been the most research support for dopamine and serotonin pathways:
- Dopamine: Dopamine is a monoamine neurotransmitter that has been found to promote exploratory behavior.[19] Dopaminergic pathways have been specifically correlated with the extraversion trait of the Five Factor Model of Personality.[15] The monoamine oxidase (MAO) enzyme has a preferential affinity for dopamine, and its levels are inversely correlated with sensation seeking.[16]
- Serotonin: Serotonin is a monoamine neurotransmitter, and has been found to promote avoidance behavior through inhibitory pathways.[19] Specifically, serotonin has been associated with Neuroticism, Agreeableness, and Conscientiousness (traits defined by the Five Factor Model of Personality).[15]
Genes
Previous studies show that genes account for at most 50 percent of a given trait.[1] However, it is widely accepted that variance in gene sequence affect behavior, and genes are a significant risk factor for personality disorders.[20] With the growing interest in using molecular genetics in tracing the biological basis of personality,[8] there may be more gene-trait links found in the future.
Varying polymorphisms and sequence repeats in the gene for dopamine receptor D4 and serotonin transporter gene 5-HTTLPR, have both been found to influence the extraversion trait in adults. Specifically, study participants with at least one copy of the 7-repeat variant of the dopamine receptor D4 gene had higher scores of self-reported extraversion.[8] This suggests that dopamine and serotonin interact to regulate the conflicting behavioral traits of careless exploration vs. cautious inhibition.[19]
Synaptic plasticity
Synaptic plasticity refers to the ability of neurons to strengthen or weaken the connections between them. According to Hebbian theory, these connections are strengthened and maintained through repeated stimulation between neurons. Specifically, there is an emphasis on long-term potentiation (LTP), which is the prolonged strengthening of synaptic connections that facilitate learning from experience.
On a larger scale, there are many pathways and brain regions that are interdependent and contribute to a cohesive, stable personality. For example, the amygdala and hippocampus of the limbic system mediate emotional intensity and consolidate memory of these experiences. But the basic mechanism by which these pathways and brain regions perform these functions, is synaptic plasticity. Ultimately, it boils down to this feature of neurons that allows the brain to learn from repeated experiences, retain memories, and ultimately maintain personality.[21] Joseph LeDoux, an award-winning neuroscientist, asserts that although humans share the same brain systems, it is the unique wiring of neurons that is different in each person and makes their personality.[21]
Brain imaging basis of personality
Over the past two decades, structural magnetic resonance imaging (sMRI) and functional magnetic resonance imaging (fMRI) techniques have been used to study associations between neural activations in the brain and personality traits and other cognitive, social, and emotional processes that characterize personality. Using MRI-based methods for such studies has become increasingly popular due to the non-invasive nature of MRI and the high resolution of MRI.
Structural magnetic resonance imaging
The use of structural magnetic resonance imaging (sMRI) to understand the neurobiological basis of personality and sociocognitive functioning involves assessing the relationship between individual differences in these factors and individual differences in measures of brain structure, such as gray matter volume, cortical thickness, or structural integrity of white matter tracts.
Studies have shown that brain volume is meaningfully correlated with four of the Big Five personality measures. Extraversion was associated with increased volume of medial orbitofrontal cortex, a region associated with processing reward-related stimuli. Conscientiousness was associated with increased volume in the lateral prefrontal cortex, a region involved in planning and the voluntary control of behavior. Agreeableness was associated with increased volume in regions involved in mentalizing, which is the ability to infer the intentions and mental states of other individuals. Neuroticism was associated with increased volume of brain regions associated with threat, punishment, and negative emotions. Openness/Intellect was not significantly correlated with the volume of any brain structures.[22] In another study, neuroticism was negatively correlated with the gray matter volume of the right amygdala, whereas extraversion was positively correlated with gray matter volume of the left amygdala.[23] A separate study also reported a significant association between neuroticism scores and gray matter volume of the left amygdala.[24] In one MRI study,[25] Novelty Seeking correlated with increased grey matter volume in regions of the cingulate cortex, Harm Avoidance correlated with decreased grey matter volume in the orbitofrontal, occipital, and parietal cortex. Reward Dependence correlated with decreased grey matter volume in the caudate nucleus.
A separate but similar line of research has used diffusion tensor imaging to measure the structural integrity of white matter in the brain. One study has shown that neuroticism is negatively correlated with the structural integrity of white matter tracts that connect various brain regions, such as the prefrontal cortex, parietal cortex, amygdala, and other regions in the subcortex. On the other hand, Openness and Agreeableness are positively associated with the structural integrity of these white matter tracts. Openness was also positively associated with the structural integrity of white matter interconnecting dorsolateral prefrontal cortex in both hemispheres.[26]
Functional magnetic resonance imaging
Functional magnetic resonance imaging (fMRI) involves the indirect measurement of neural activity by measuring disturbances in local magnetic fields in the brain. These local disturbances are linked to differential amounts of blood flow to the brain, which is linked to neural activity. Early work using fMRI has studied whether individual differences in personality traits and sociocognitive functioning are associated with individual differences in neural activations in certain brain regions during certain tasks. Such studies have demonstrated associations between single brain regions’ neural responses to certain tasks and individual differences in a wide range of sociocognitive functioning, such as approach/avoidance behavior,[27] sensitivity to rejection,[28] conceptions of the self,[29][30] and susceptibility to persuasive messages.[31] A small collection of fMRI studies have also demonstrated a significant relationship between brain responses to certain tasks and personality survey measures, such as extraversion and neuroticism.[32][33]
Over time, neuroscience researchers have recognized that brain regions do not operate in isolation. In fact, the synchronization of firing rates of neurons across different brain regions helps mediate the integration and processing of information across the brain.[34][35] Thus, studies relating neural activation in single regions to personality measures and associated sociocognitive functioning ignore information about how personality and sociocognitive functioning relate to neural activations across multiple regions in the brain. For example, it is unlikely that neural activation in a single brain region is unilaterally associated with individual differences in personality measures, such as the tendency to down-regulate negative emotions. However, the functional connectivity, or the synchronization of neural activity, between two brain regions can be related to individual differences in personality and sociocognitive functioning. For example, one study found that in an emotion regulation task, coupling of neural responses in the amygdala and the prefrontal cortex was significantly associated with more successful regulation of negative emotions.[36] Other studies shown that neuroticism is associated with relatively low functional connectivity between amygdala and anterior cingulate cortex during a variety of tasks, such as viewing negative emotional stimuli[37][38] and during a classical conditioning reward task.[39]
Resting-state functional connectivity
Functional connectivity can also be measured at rest, during which individuals are not explicitly engaged in any task.[40] These resting-state functional connectivities can also be related to personality measures and other sociocognitive functioning. For instance, one study found that functional connectivity patterns originating from the amygdala are predictive of neuroticism and extraversion scores.[41] However, personality measures and sociocognitive functioning are not subserved solely by the functional connectivity between two given brain regions. Indeed, examining functional connectivity across the brain may shed more light on the neurobiological basis of personality and sociocognitive functioning.[42] For example, a recent line of research has demonstrated that individual differences in functional connectomes, which are characterized by patterns of spontaneous synchronization of neural activations across the entire brain, are predictive of individual differences in personality and sociocognitive functioning, such as openness to experience,[43] fluid intelligence,[44] and trait levels of paranoia.[45] The use of functional connectomes to predict individual differences is known as “functional connectome fingerprinting” and allows researcher to construct models of personality and sociocognitive functioning based on neural activity across the whole brain rather than within single regions (if using neural activations) or single pairs of regions (if using functional connectivity).[46]
Graph theory-based analysis
Functional connectomes can be distilled into constituent intrinsic brain networks that are present during sleep, at rest, and during tasks.[47] These brain networks can also reliably be mapped onto cognitive systems.[48][49] The default mode network, for example, is composed of regions such as the medial prefrontal cortex, angular gyrus, temporoparietal junction, and the hippocampus, to name a few. One study has shown that Extraversion and Agreeableness are positively correlated with overall neural activity in the default mode network.[50] Assessing the relationship between neural activity in brain networks and personality traits is an important first step for identifying where the neurobiological basis of personality traits may be localized. However, this approach does not offer a complete mechanistic explanation of how and why individual differences in these brain networks are related to individual differences in personality.[42] To address this gap, neuroscience researchers have begun to leverage graph theoretical approaches to better understand characteristics of these brain networks, such as their assortativity, efficiency, and modularity. For example, one study has demonstrated that individual differences in anxiety-related harm avoidance behavior was associated with relatively low efficiency (i.e., high path length) in the insular-opercular brain network at rest. This finding suggests that trait anxiety may be associated with relatively slow and inefficient transfer of information within the insular-opercular brain network.[51] Another study used a graph theoretical approach to demonstrate that high trait impulsivity was associated with relatively high modularity of resting-state brain networks, such that brain networks exhibited relatively high within-system density of functional connectivity but relatively low between-system density of functional connectivity.[52] A separate study has also demonstrated that high Conscientiousness is associated with high local clustering and high betweenness centrality within the default mode network and the fronto-parietal network (FPN).[53] Given the role of the FPN in cognitive control, these findings suggest that people high on Conscientiousness may exhibit higher cognitive control. Furthermore, heightened interconnectivity within the DMN also provides convergent evidence that highly conscientious individuals may be adept at high-level cognitive tasks, such as complex planning, given that the DMN is strongly associated with high-level executive function and working memory.[53]
References
- LeDoux, J. (2003). "The Self". Annals of the New York Academy of Sciences. 1001 (1): 295–304. doi:10.1196/annals.1279.017. PMID 14625368. S2CID 39483722.
- Davidson, R. J. (2001). "Toward a biology of personality and emotion". Ann N Y Acad Sci. 935 (1): 191–207. Bibcode:2001NYASA.935..191D. CiteSeerX 10.1.1.1069.2394. doi:10.1111/j.1749-6632.2001.tb03481.x. PMID 11411166. S2CID 6175240.
- Corr, Philip J.; Perkins, Adam M. (2006). "The role of theory in the psychophysiology of personality: From Ivan Pavlov to Jeffrey Gray". International Journal of Psychophysiology. 62 (3): 367–376. doi:10.1016/j.ijpsycho.2006.01.005. ISSN 0167-8760. PMID 16515814.
- Fowles, Don (2006). "Chapter 2: Jeffrey Gray's Contributions to Theories of Anxiety, Personality, and Psychopathology". In Canli, Turhan (ed.). Biology of personality and individual differences. Guilford Press. ISBN 978-1593852528..
- The Journal of Mental Health, July 1951, Vol. XCVII, "The Inheritance of Neuroticism: An Experimental Study", H. J. Eysenck and D. B. Prell, p. 402.
- "Some Implications of the Eysenck-Prell Study of "The Inheritance of Neuroticism:" A Critique". www.ets.org. Retrieved 2020-02-29.
- Canli, Turhan (2006). "Chapter 5: Genomic Imaging of Extraversion". In Canli, Turhan (ed.). Biology of personality and individual differences. Guilford Press. ISBN 978-1593852528..
- Canli, Turhan (2006). "Chapter 1: Introduction". In Canli, Turhan (ed.). Biology of personality and individual differences. Guilford Press. ISBN 978-1593852528..
- DeYoung, Colin G. (2010). "Personality Neuroscience and the Biology of Traits". Social and Personality Psychology Compass. 4 (12): 1165–1180. doi:10.1111/j.1751-9004.2010.00327.x. ISSN 1751-9004. S2CID 15018241.
- Corr, Philip J; Mobbs, Dean (2018-05-25). "From Epiphenomenon to Biologically Important Phenomena". Personality Neuroscience. 1: e1. doi:10.1017/pen.2017.1. ISSN 2513-9886. PMC 7219691. PMID 32435724.
- DeYoung, Colin G; Peterson, Jordan B; Higgins, Daniel M (2002-09-01). "Higher-order factors of the Big Five predict conformity: Are there neuroses of health?". Personality and Individual Differences. 33 (4): 533–552. doi:10.1016/S0191-8869(01)00171-4. ISSN 0191-8869.
- DeYoung, Colin G. (2006). "Higher-order factors of the Big Five in a multi-informant sample". Journal of Personality and Social Psychology. 91 (6): 1138–1151. doi:10.1037/0022-3514.91.6.1138. ISSN 1939-1315. PMID 17144770. S2CID 35478689.
- Jang, Kerry L.; McCrae, Robert R.; Angleitner, Alois; Riemann, Rainer; Livesley, W. John (1998). "Heritability of facet-level traits in a cross-cultural twin sample: Support for a hierarchical model of personality". Journal of Personality and Social Psychology. 74 (6): 1556–1565. doi:10.1037/0022-3514.74.6.1556. ISSN 1939-1315. PMID 9654759.
- DeYoung, Colin G. (2013). "The neuromodulator of exploration: A unifying theory of the role of dopamine in personality". Frontiers in Human Neuroscience. 7: 762. doi:10.3389/fnhum.2013.00762. ISSN 1662-5161. PMC 3827581. PMID 24294198.
- DeYoung, Colin G. (2010). "Personality Neuroscience and the Biology of Traits". Social and Personality Psychology Compass. 4 (12): 1165–1180. doi:10.1111/j.1751-9004.2010.00327.x. ISSN 1751-9004. S2CID 15018241.
- Zuckerman, Marvin (2006). "Chapter 3: Biosocial Bases of Sensation Seeking". In Canli, Turhan (ed.). Biology of personality and individual differences. Guilford Press. ISBN 978-1593852528..
- Mehta, Pranjal; Gosling, Samuel (2006). "Chapter 20: How Can Animal Studies Contribute to Research on the Biological Bases of Personality". In Canli, Turhan (ed.). Biology of personality and individual differences. Guilford Press. ISBN 978-1593852528..
- Bell, A.M., & Aubin-Horth, N. (2010). What can whole genome expression data tell us about the ecology and evolution of personality?. Philosophical Transactions of the Royal Society B: Biological Sciences. Retrieved September 10, 2014, from http://rstb.royalsocietypublishing.org/content/365/1560/4001.full.pdf+html.
- Ebstein, Richard P.; Auerbach, Judith G. (2002). "Dopamine D4 receptor and serotonin transporter promoter polymorphisms and temperament in early childhood". Molecular Genetics and the Human Personality: 137–149.
- Whittle, Sarah; Allen, Nicholas B.; Lubman, Dan I.; Yücel, Murat (2006). "The neurobiological basis of temperament: Towards a better understanding of psychopathology". Neuroscience & Biobehavioral Reviews. Elsevier BV. 30 (4): 511–525. doi:10.1016/j.neubiorev.2005.09.003. ISSN 0149-7634. PMID 16289282. S2CID 2406240.
- LeDoux, J. E. (2003). Synaptic Self: How Our Brains Become Who We Are: Penguin Books.
- DeYoung, Colin G.; Hirsh, Jacob B.; Shane, Matthew S.; Papademetris, Xenophon; Rajeevan, Nallakkandi; Gray, Jeremy R. (June 2010). "Testing Predictions From Personality Neuroscience: Brain Structure and the Big Five". Psychological Science. 21 (6): 820–828. doi:10.1177/0956797610370159. ISSN 0956-7976. PMC 3049165. PMID 20435951.
- Omura, Kazufumi; Todd Constable, R.; Canli, Turhan (November 2005). "Amygdala gray matter concentration is associated with extraversion and neuroticism". NeuroReport. 16 (17): 1905–1908. doi:10.1097/01.wnr.0000186596.64458.76. ISSN 0959-4965. PMID 16272876. S2CID 19309461.
- Koelsch, Stefan; Skouras, Stavros; Jentschke, Sebastian (2013-11-27). "Neural Correlates of Emotional Personality: A Structural and Functional Magnetic Resonance Imaging Study". PLOS ONE. 8 (11): e77196. Bibcode:2013PLoSO...877196K. doi:10.1371/journal.pone.0077196. ISSN 1932-6203. PMC 3842312. PMID 24312166.
- Gardini, Simona; Cloninger, C. Robert; Venneri, Annalena (2009). "Individual differences in personality traits reflect structural variance in specific brain regions". Brain Research Bulletin. 79 (5): 265–270. doi:10.1016/j.brainresbull.2009.03.005. ISSN 0361-9230. PMID 19480986. S2CID 25490518.
- Xu, Jiansong; Potenza, Marc N. (January 2012). "White matter integrity and five-factor personality measures in healthy adults". NeuroImage. 59 (1): 800–807. doi:10.1016/j.neuroimage.2011.07.040. PMC 3195960. PMID 21840401.
- Gray, Jeremy R.; Burgess, Gregory C.; Schaefer, Alexandre; Yarkoni, Tal; Larsen, Randy J.; Braver, Todd S. (2005-06-01). "Affective personality differences in neural processing efficiency confirmed using fMRI". Cognitive, Affective, & Behavioral Neuroscience. 5 (2): 182–190. doi:10.3758/CABN.5.2.182. ISSN 1531-135X. PMID 16180624.
- Eisenberger, Naomi I.; Lieberman, Matthew D. (2004-07-01). "Why rejection hurts: a common neural alarm system for physical and social pain". Trends in Cognitive Sciences. 8 (7): 294–300. doi:10.1016/j.tics.2004.05.010. ISSN 1364-6613. PMID 15242688. S2CID 15893740.
- Ma, Yina; Bang, Dan; Wang, Chenbo; Allen, Micah; Frith, Chris; Roepstorff, Andreas; Han, Shihui (2012-09-05). "Sociocultural patterning of neural activity during self-reflection". Social Cognitive and Affective Neuroscience. 9 (1): 73–80. doi:10.1093/scan/nss103. ISSN 1749-5024. PMC 3871729. PMID 22956678.
- Ray, Rebecca D.; Shelton, Amy L.; Hollon, Nick G.; Matsumoto, David; Frankel, Carl B.; Gross, James J.; Gabrieli, John D.E. (2010-06-01). "Interdependent self-construal and neural representations of self and mother". Social Cognitive and Affective Neuroscience. 5 (2–3): 318–323. doi:10.1093/scan/nsp039. ISSN 1749-5024. PMC 2894675. PMID 19822601.
- Falk, Emily B.; Berkman, Elliot T.; Whalen, Danielle; Lieberman, Matthew D. (2011). "Neural activity during health messaging predicts reductions in smoking above and beyond self-report". Health Psychology. 30 (2): 177–185. doi:10.1037/a0022259. ISSN 1930-7810. PMC 3059382. PMID 21261410.
- Eisenberger, Naomi I.; Lieberman, Matthew D.; Satpute, Ajay B. (2005-06-01). "Personality from a controlled processing perspective: An fMRI study of neuroticism, extraversion, and self-consciousness". Cognitive, Affective, & Behavioral Neuroscience. 5 (2): 169–181. doi:10.3758/CABN.5.2.169. ISSN 1531-135X. PMID 16180623.
- Canli, Turhan; Sivers, Heidi; Whitfield, Susan L.; Gotlib, Ian H.; Gabrieli, John D. E. (2002-06-21). "Amygdala Response to Happy Faces as a Function of Extraversion". Science. 296 (5576): 2191. doi:10.1126/science.1068749. ISSN 0036-8075. PMID 12077407. S2CID 45096216.
- Fries, Pascal (2005-10-01). "A mechanism for cognitive dynamics: neuronal communication through neuronal coherence". Trends in Cognitive Sciences. 9 (10): 474–480. doi:10.1016/j.tics.2005.08.011. ISSN 1364-6613. PMID 16150631. S2CID 6275292.
- Fries, Pascal (2015-10-07). "Rhythms for Cognition: Communication through Coherence". Neuron. 88 (1): 220–235. doi:10.1016/j.neuron.2015.09.034. ISSN 0896-6273. PMC 4605134. PMID 26447583.
- Lee, Hyejeen; Heller, Aaron S.; van Reekum, Carien M.; Nelson, Brady; Davidson, Richard J. (2012-09-01). "Amygdala–prefrontal coupling underlies individual differences in emotion regulation". NeuroImage. 62 (3): 1575–1581. doi:10.1016/j.neuroimage.2012.05.044. ISSN 1053-8119. PMC 3408571. PMID 22634856.
- Cremers, Henk R.; Demenescu, Liliana R.; Aleman, André; Renken, Remco; van Tol, Marie-José; van der Wee, Nic J. A.; Veltman, Dick J.; Roelofs, Karin (2010-01-01). "Neuroticism modulates amygdala—prefrontal connectivity in response to negative emotional facial expressions". NeuroImage. 49 (1): 963–970. doi:10.1016/j.neuroimage.2009.08.023. ISSN 1053-8119. PMID 19683585. S2CID 6842533.
- Gentili, Claudio; Cristea, Ioana Alina; Ricciardi, Emiliano; Vanello, Nicola; Popita, Cristian; David, Daniel; Pietrini, Pietro (2017-06-01). "Not in one metric: Neuroticism modulates different resting state metrics within distinctive brain regions". Behavioural Brain Research. 327: 34–43. doi:10.1016/j.bbr.2017.03.031. hdl:11568/857552. ISSN 0166-4328. PMID 28342970. S2CID 19925624.
- Schweckendiek, Jan; Stark, Rudolf; Klucken, Tim (2016). "Neuroticism and extraversion moderate neural responses and effective connectivity during appetitive conditioning". Human Brain Mapping. 37 (8): 2992–3002. doi:10.1002/hbm.23221. ISSN 1097-0193. PMC 6867409. PMID 27132706.
- Raichle, Marcus E.; Snyder, Abraham Z. (2007-10-01). "A default mode of brain function: A brief history of an evolving idea". NeuroImage. 37 (4): 1083–1090. doi:10.1016/j.neuroimage.2007.02.041. ISSN 1053-8119. PMID 17719799. S2CID 3380973.
- Aghajani, Moji; Veer, Ilya M.; van Tol, Marie-José; Aleman, André; van Buchem, Mark A.; Veltman, Dick J.; Rombouts, Serge A. R. B.; van der Wee, Nic J. (June 2014). "Neuroticism and extraversion are associated with amygdala resting-state functional connectivity". Cognitive, Affective, & Behavioral Neuroscience. 14 (2): 836–848. doi:10.3758/s13415-013-0224-0. ISSN 1530-7026. PMID 24352685. S2CID 207698821.
- Tompson, Steven H.; Falk, Emily B.; Vettel, Jean M.; Bassett, Danielle S. (July 2018). "Network Approaches to Understand Individual Differences in Brain Connectivity: Opportunities for Personality Neuroscience". Personality Neuroscience. 1. doi:10.1017/pen.2018.4. ISSN 2513-9886. PMC 6133307. PMID 30221246.
- Dubois, Julien; Galdi, Paola; Han, Yanting; Paul, Lynn K.; Adolphs, Ralph (2018-07-05). "Resting-State Functional Brain Connectivity Best Predicts the Personality Dimension of Openness to Experience". Personality Neuroscience. 1: e6. doi:10.1017/pen.2018.8. ISSN 2513-9886. PMC 6138449. PMID 30225394.
- Finn, Emily S; Shen, Xilin; Scheinost, Dustin; Rosenberg, Monica D; Huang, Jessica; Chun, Marvin M; Papademetris, Xenophon; Constable, R Todd (November 2015). "Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity". Nature Neuroscience. 18 (11): 1664–1671. doi:10.1038/nn.4135. ISSN 1097-6256. PMC 5008686. PMID 26457551.
- Finn, Emily S.; Corlett, Philip R.; Chen, Gang; Bandettini, Peter A.; Constable, R. Todd (December 2018). "Trait paranoia shapes inter-subject synchrony in brain activity during an ambiguous social narrative". Nature Communications. 9 (1): 2043. Bibcode:2018NatCo...9.2043F. doi:10.1038/s41467-018-04387-2. ISSN 2041-1723. PMC 5966466. PMID 29795116.
- Shen, Xilin; Finn, Emily S.; Scheinost, Dustin; Rosenberg, Monica D.; Chun, Marvin M.; Papademetris, Xenophon; Constable, R. Todd (March 2017). "Using connectome-based predictive modeling to predict individual behavior from brain connectivity". Nature Protocols. 12 (3): 506–518. doi:10.1038/nprot.2016.178. ISSN 1750-2799. PMC 5526681. PMID 28182017.
- Raichle, Marcus E. (January 2011). "The Restless Brain". Brain Connectivity. 1 (1): 3–12. doi:10.1089/brain.2011.0019. ISSN 2158-0014. PMC 3621343. PMID 22432951.
- Power, Jonathan D.; Cohen, Alexander L.; Nelson, Steven M.; Wig, Gagan S.; Barnes, Kelly Anne; Church, Jessica A.; Vogel, Alecia C.; Laumann, Timothy O.; Miezin, Fran M.; Schlaggar, Bradley L.; Petersen, Steven E. (November 2011). "Functional Network Organization of the Human Brain". Neuron. 72 (4): 665–678. doi:10.1016/j.neuron.2011.09.006. PMC 3222858. PMID 22099467.
- Thomas Yeo, B. T.; Krienen, Fenna M.; Sepulcre, Jorge; Sabuncu, Mert R.; Lashkari, Danial; Hollinshead, Marisa; Roffman, Joshua L.; Smoller, Jordan W.; Zöllei, Lilla; Polimeni, Jonathan R.; Fischl, Bruce (September 2011). "The organization of the human cerebral cortex estimated by intrinsic functional connectivity". Journal of Neurophysiology. 106 (3): 1125–1165. Bibcode:2011NatSD...2E0031H. doi:10.1152/jn.00338.2011. ISSN 0022-3077. PMC 3174820. PMID 21653723.
- Sampaio, Adriana; Soares, José Miguel; Coutinho, Joana; Sousa, Nuno; Gonçalves, Óscar F. (November 2014). "The Big Five default brain: functional evidence". Brain Structure and Function. 219 (6): 1913–1922. doi:10.1007/s00429-013-0610-y. hdl:10316/93845. ISSN 1863-2653. PMID 23881294. S2CID 18141160.
- Markett, Sebastian; Montag, Christian; Melchers, Martin; Weber, Bernd; Reuter, Martin (December 2016). "Anxious personality and functional efficiency of the insular-opercular network: A graph-analytic approach to resting-state fMRI". Cognitive, Affective, & Behavioral Neuroscience. 16 (6): 1039–1049. doi:10.3758/s13415-016-0451-2. ISSN 1530-7026. PMID 27515174.
- Davis, F. Caroline; Knodt, Annchen R.; Sporns, Olaf; Lahey, Benjamin B.; Zald, David H.; Brigidi, Bart D.; Hariri, Ahmad R. (2013-06-01). "Impulsivity and the Modular Organization of Resting-State Neural Networks". Cerebral Cortex. 23 (6): 1444–1452. doi:10.1093/cercor/bhs126. ISSN 1047-3211. PMC 3643719. PMID 22645253.
- Toschi, Nicola; Riccelli, Roberta; Indovina, Iole; Terracciano, Antonio; Passamonti, Luca (2018-05-25). "Functional Connectome of the Five-Factor Model of Personality". Personality Neuroscience. 1: e2. doi:10.1017/pen.2017.2. ISSN 2513-9886. PMC 6171528. PMID 30294715.