Bis(acetonitrile)palladium dichloride
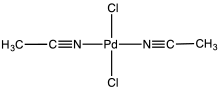
Bis(acetonitrile)palladium dichloride is the coordination complex with the formula PdCl2(NCCH3)2. It is the adduct of two acetonitrile ligands with palladium(II) chloride. It is a yellow-brown solid that is soluble in organic solvents. The compound is a reagent and a catalyst for reactions that require soluble Pd(II).[1] The compound is similar to bis(benzonitrile)palladium dichloride. It reacts with 1,5-cyclooctadiene to give dichloro(1,5‐cyclooctadiene)palladium.
 | |
2PdCl2-from-xtal-3D-bs-17.png.webp) | |
2.jpg.webp) | |
| Names | |
|---|---|
| Other names
palladium dichloride bis(acetonitrile), bis(acetonitrile)dichloropalladium | |
| Identifiers | |
3D model (JSmol) |
|
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| C4H6Cl2N2Pd | |
| Molar mass | 259.43 g·mol−1 |
| Appearance | yellow-brown |
| Melting point | 129–131 °C (264–268 °F; 402–404 K) |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H301, H311, H330 | |
| P260, P261, P264, P270, P271, P273, P280, P284, P301+P310, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P310, P311, P312, P320, P321, P322, P330, P332+P313, P337+P313, P361, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Carretero, Juan Carlos; Arrayas, Ramon Gomez (2008). "Dichloro bis(acetonitrile) palladium". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00908. ISBN 978-0471936237.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.