Bisindolylmaleimide
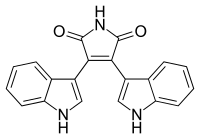
Bisindolylmaleimide is an organic compound that forms the core chemical structure of a variety of biologically active compounds.[1] This core structure includes a central maleimide group with two indole groups attached.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3,4-Di(1H-indol-2-yl)-1H-pyrrole-2,5-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H13N3O2 | |
| Molar mass | 327.343 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Examples of bisindolylmaleimide derivatives include:
References
- Faul, Margaret M.; Winneroski, Leonard L.; Krumrich, Christine A. (1998). "A New, Efficient Method for the Synthesis of Bisindolylmaleimides". The Journal of Organic Chemistry. 63 (17): 6053. doi:10.1021/jo980513c. PMID 11672217.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.