Bitartrate
Bitartrate is an anion which is the conjugate base of tartaric acid. It may also refer to any salt or monoester of tartaric acid.
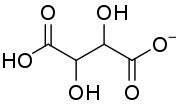 | |
| Names | |
|---|---|
| Preferred IUPAC name | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| 3905887[1][3] | |
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| Properties | |
| C4H5O6− | |
| Molar mass | 149.079 g·mol−1 |
| Conjugate acid | Tartaric acid |
| Conjugate base | Tartrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Some examples of bitartrate salts include:
References
- "3-carboxy-2,3-dihydroxypropanoate (CHEBI:48929)". www.ebi.ac.uk. 25 June 2014. Retrieved 27 January 2019.
- "3-Carboxy-2,3-dihydroxypropanoate | C4H5O6 | ChemSpider". www.chemspider.com. Retrieved 27 January 2019.
- "Hydrogen tartrate". pubchem.ncbi.nlm.nih.gov. Retrieved 27 January 2019.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.