Biuret
Biuret is a chemical compound with the chemical formula HN(CONH2)2. It is a white solid that is soluble in hot water. A variety of organic derivatives are known. The term "biuret" also describes a family of organic compounds with the chemical formula R1R2N−C(=O)−N(R3)−C(=O)−NR4R5, where R1, R2, R3, R4 and R5 are hydrogen, organyl or other groups. Also known as carbamylurea, it results from the condensation of two equivalents of urea. It is a common undesirable impurity in urea-based fertilizers, as biuret is toxic to plants.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Imidodicarbonic diamide[1] | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 1703510 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.236 |
| EC Number |
|
| 49702 | |
| KEGG | |
| MeSH | Biuret |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| HN(CONH2)2 | |
| Molar mass | 103.081 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| Density | 1.467 g/cm3 |
| Melting point | 190 °C (decomposes) |
| Thermochemistry | |
Heat capacity (C) |
131.3 J/(mol·K) |
Std molar entropy (S⦵298) |
146.1 J/(mol·K) |
Std enthalpy of formation (ΔfH⦵298) |
(−565.8) – (−561.6) kJ/mol |
Std enthalpy of combustion (ΔcH⦵298) |
(−940.1) – (−935.9) kJ/mol |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P305+P351+P338 | |
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation and structure
The parent compound can be prepared by heating urea at 150 °C for ~6 hours until it gets slightly cloudy, then recrystallizing from water. After that, it can be recrystallized repeatedly from 2% sodium hydroxide solution and water to finally get base-free crystalline needles of the monohydrate which are free of cyanuric acid. While heating, a lot of ammonia is expelled:[3]
- 2 CO(NH2)2 → HN(CONH2)2 + NH3
Under related conditions, pyrolysis of urea affords triuret O=C(−N(H)−C(=O)−NH2)2.[3] In general, organic biurets (those with alkyl or aryl groups in place of one or more H atoms) are prepared by trimerization of isocyanates. For example, the trimer of 1,6-hexamethylene diisocyanate is also known as HDI-biuret.
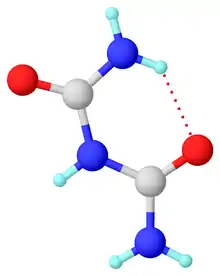
In the anhydrous form, the molecule is planar and unsymmetrical in the solid state owing to intramolecular hydrogen bonding. The terminal C–N distances of 1.327 and 1.334 Å are shorter than the internal C–N distances of 1.379 and 1.391 Å. The C=O bond distances 1.247 and 1.237 Å. It crystallizes from water as the monohydrate.[4]
Applications
Biuret is also used as a non-protein nitrogen source in ruminant feed,[5] where it is converted into protein by gut microorganisms.[6] It is less favored than urea, due to its higher cost and lower digestibility[7] but the latter characteristic also slows down its digestion and so decreases the risk of ammonia toxicity.[7][8]
Biuret test
The biuret test is a chemical test for proteins and polypeptides. It is based on the biuret reagent, a blue solution that turns violet upon contact with proteins, or any substance with peptide bonds. The test and reagent do not actually contain biuret; they are so named because both biuret and proteins have the same response to the test.
History
Biuret was first prepared and studied by Gustav Heinrich Wiedemann (1826–1899) for his doctoral dissertation, which was submitted in 1847. His findings were reported in several articles.[9][10][11][12]
Related compounds
- Cyanuric acid
- Allophanic acid, the carboxylic acid derivative of biuret
References
- Favre, Henri A.; Powell, Warren H. (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. p. 866. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- Scifinder, version 2007.1; Chemical Abstracts Service: Columbus, OH; RN 108-19-0 (accessed June 15, 2012)
- Meessen, J. H.; Petersen, H. "Urea". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_333.
- E. W. Hughes; H. Yakel; H. C. Freeman (1961). "The Crystal Structure of Biuret Hydrate". Acta Crystallogr. 14 (4): 345–352. doi:10.1107/S0365110X61001194.
- Beef cattle feed, Encyclopædia Britannica Online
- Kunkle, B.; Fletcher, J.; Mayo, D. (2013). "Florida Cow-Calf Management, 2nd Edition - Feeding the Cow Herd". IFAS Extension, University of Florida. Publication #AN117. Archived from the original on 2019-05-13. Retrieved 2008-01-15.
- Oltjen, R. R.; Williams, E. E.; Slyter, L. L.; Richardson, G. V. (1969). "Urea versus biuret in a roughage diet for steers". Journal of Animal Science. 29 (5): 816–822. doi:10.2527/jas1969.295816x. PMID 5391979. Archived from the original on 2021-01-12. Retrieved 2013-10-22.
- Fonnesbeck, Paul V.; Kearl, Leonard C.; Harris, Lorin E. (1975). "Feed Grade Biuret as a Protein Replacement for Ruminants. A Review". Journal of Animal Science. Oxford University Press (OUP). 40 (6): 1150–1184. doi:10.2527/jas1975.4061150x. ISSN 0021-8812.
- Wiedemann, G. (1848). "Ueber ein neues Zersetzungsproduct des Harnstoffs" [On a new decomposition product of urea]. Annalen der Physik. 150 (5): 67–84. Bibcode:1848AnP...150...67W. doi:10.1002/andp.18491500508.
- Wiedemann, G. (1847). "Neues Zersetzungsproduct des Harnstoffs" [New decomposition product of urea]. Journal für Praktische Chemie. 42 (3–4): 255–256. doi:10.1002/prac.18470420134. This notice reports that biuret reacts with alkaline copper sulfate to produce a red solution – the so-called "Biuret test"
- Wiedemann, G. (1848). "Ueber eine neue, aus dem Harnstoff entstehende Verbindung" [On a new compound arising from urea]. Journal für Praktische Chemie. 43 (5): 271–280. doi:10.1002/prac.18480430133.
- Wiedemann, G. (1848). "Biuret. Zersetzungsprodukt des Harnstoffs" [Biuret: decomposition product of urea]. Justus Liebig's Annalen der Chemie. 68 (3): 323–326. doi:10.1002/jlac.18480680318.