Botryosphaeran
Botryosphaeran is an exopolysaccharide (EPS) produced by the ascomyceteous fungus Botryosphaeria rhodina.[1][2] Characterization of the chemical structure of botryosphaeran showed this EPS to be a (1→3)(1→6)-β-D-glucan.[3] This particular β-glucan can be produced by several strains of Botryosphaeria rhodina that include: MAMB-05,[1] DABAC-P82,[4] and RCYU 30101.[5] Botryosphaeran exhibits interesting rheological properties and novel biological functions including hypoglycaemia, hypocholesterolaemia, anti-atheroslerosis and anti-cancer activity, with potential commercial applications. Three cosmetic products formulated with botryosphaeran have been developed to promote skin health and treat skin conditions for future intended commercialization purposes.
 | |
| Identifiers | |
|---|---|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
History
The ascomycete and filamentous fungus, Botryosphaeria rhodina (strain MAMB-05), was isolated from a canker on the trunk of a eucalypt tree, and was molecularly characterized by sequencing the Internal Transcribed Spacer (ITS) region of rDNA.[6][7]
The β-glucan, botryosphaeran, was discovered accidentally in 1994[1] while cultivating Botryosphaeria rhodina MAMB-05 on nutrient media containing glucose to produce the enzyme, laccase. This fungal isolate produces a constitutive laccase that could be induced to higher enzyme titers by various lignin-like aromatic compounds, and especially veratryl alcohol.[1][8] The fungus was found to be ligninolytic.[9][10]
Botryosphaeran is secreted by the fungus during growth and appears in the fermentation broth where its presence causes an increase in the broth's viscosity. It can easily be extracted from the broth by precipitation methods.[1] Veratryl alcohol, however, suppresses the formation of botryosphaeran.[11]
Production and isolation
Botryosphaeran is produced under submerged fermentation conditions when Botryosphaeria rhodina MAMB-05 is grown on nutrient media containing glucose and mineral salts.[1] Extracting the fermentation broth with alcohol causes the EPS (botryosphaeran) to precipitate from solution, and this can be separated by centrifugation or filtration.
The precipitate recovered can be lyophilized to a white fibrous material that is sparingly soluble in water. Alternatively, the recovered precipitate is resolubilized in water (gentle heating with stirring) to form a viscous solution that forms a firm gel when cooled to 5 °C. Solubilization of botryosphaeran can be enhanced through chemical derivatization with various functional groups.
The influence of the composition of the nutrient medium,[12] including nitrogen,[13] phosphate,[13] minerals, supplements (soybean oil, Tween 80),[14] and the carbon source (carbohydrates),[12][15] is important in enhancing the production of botryosphaeran and biomass during fermentation by Botryosphaeria rhodina MAMB-05.
Catabolite repression,[16] and the presence of β-glucan-hydrolyzing enzymes that attack botryosphaeran[17] during the fermentation process are critical and limit the production of botryosphaeran.
Statistical factorial design methods, such as the response surface methodology (RSM),[18][19] are effective in investigating complex fermentation parameters and their interactions to optimize metabolite production by microorganisms. RSM assists in defining the effects and interactions of the physiological factors playing a role in biotechnological processes in the production of microbial metabolites including exopolysaccharides such as β-glucans. Statistical methodologies reduce the number of experiments to provide sufficient information for statistically acceptable results.
The validation of the fermentation parameters by statistical factorial design improved botryosphaeran production by Botryosphaeria rhodina MAMB-05[13][14] over unoptimized conditions.[12]
Botryosphaeria rhodina MAMB-05 when grown on nutrient media containing different carbohydrate substrates produces a family of botryosphaerans.[12][15] These β-glucans differ only in the extent and frequency of side-chain substituents.
Botryosphaeran production can be enhanced when Botryosphaeria rhodina MAMB-05 is cultivated on glucose media containing soybean oil and the surfactant, Tween 80.[14]
The most attractive feature for the commercialization of botryosphaeran is the ease by which it can be produced by simple fermentation processes on low-cost nutrient media, and its subsequent isolation through precipitation with ethanol,[3][12][13] which all takes place on a time-scale of days compared to other commercial β-glucans available on the market. The latter are extracted from fungal fruiting bodies (mushrooms, fungal brackets), spent Brewers yeast, and cereal grains (barley, oat) that can take weeks-to-months to prepare.
The mycelium of Botryosphaeria rhodina MAMB-05 is a rich source of β-glucans.[20]
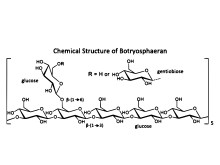
Chemical structure
The chemical structure of botryosphaeran was first described[3] in 2003, and was determined using the methods: methylation analysis, Smith degradation, Gas Chromatography-Mass Spectroscopy (GC-MS) and 13C NMR.
Hydrolysis
Total acid hydrolysis of botryosphaeran produces only D-glucose,[1][3] while partial acid hydrolysis[3] and enzymatic hydrolysis[21][22][23] produces a series of homologous gluco-oligosaccharides of different degrees of polymerization, which can be analyzed by High Performance Liquid Chromatography (HPLC).
Enzymatic digestion of botryosphaeran under controlled conditions employing the enzymes: β-(1→3)-glucanases and β-(1→6)-glucanases from Botryosphaeria rhodina MAMB-05,[24]Trichoderma harzianum Rifai,[24] and Aureobasidium pullulans 1WA1,[23] produces a mixed series of β-(1→3)- and β-(1→6)- linked gluco-oligosaccharides[21][22][23] that can serve as prebiotics.
Enzymes hydrolyzing botryosphaeran can be obtained by cultivating Botryosphaeria rhodina MAMB-05, Trichoderma harzianum Rifai and Aureobasidium pullulans 1WA1 on nutrient media containing either botryosphaeran,[23][24] or the biomass[17][24] derived from Botryosphaeria rhodina MAMB-05, which is a rich source of β-glucans.[20]
Prebiotics such as the (1→3)-linked gluco-oligosaccharides are emerging as nutraceuticals for inclusion in foods. Botryosphaeran can serve as a source of conveniently generating these oligosaccharides through enzymatic hydrolysis.[21]
Chemical structure characterization
Methylation and Smith degradation analysis revealed that botryosphaeran constituted a backbone chain made up of (1→3)-β-linked glucose residues (i.e., it is a (1→3)-β-D-glucan) with β-(1→6)-linked glucose and di-glucose (gentiobiose) side-branches located at the C-6 position of glucose along the (1→3)-linked backbone chain.[3][4] The chemical structure of botryosphaeran is a (1→3)(1→6)-β-D-glucan.[3] 13C NMR spectroscopy confirmed its structure.
The degree of branching of the family of botryosphaerans varies from 21 to 31%.[3][15] depending upon the carbohydrate source in the nutrient media during fermentation by the fungus, and this also affects the molecular weight (MW) of the botryosphaerans produced, which can be large (order of >1 x 106 Daltons)[4][15]
Botryosphaeran exists in a triple helix conformation,[25] an important structural feature in manifesting biological response modifying activities.[26][27][28]
Derivatization of botryosphaeran by carboxymethylation and sulfonylation[29][30] results in improved solubility in water, and diminishes its viscous nature in solution.
In the case of sulfonated botryosphaeran, the chemically modified polysaccharide containing sulfonate groups exhibited new biological functions: anticoagulation,[29][30] and antiviral activity against enveloped viruses such as human herpes simplex and Dengue, The latter is a mosquito-borne virus.
Related exopolysaccharides (β-glucans) from several strains of Botryosphaeria rhodina (the teleomorph Lasiodiplodia theobromae[7]) isolated from rotting tropical fruits have been described,[31][32][33] The chemical structures of three β-glucans produced were characterized; a (1→3)(1→6)-β-D-glucan with a single glucose repeat substituent (frequency of 20%),[31] an unbranched (1→6)-β-glucan named lasiodiplodan,[31][32][33] and a new (1→3)(1→6)-β-glucan with unique branches comprising gentiobiose and gentiotriose residues, but not glucose.[33]
Structural characterization of the cell wall (mycelium) of Botryosphaeria rhodina MAMB-05 revealed the presence of three different D-glucans; a linear (1→6)-β-glucan, a branched (1→3)(1→6)-β-glucan with single glucose repeat branches (frequency of 18%), and a glycogen-like (1→4)(1→6)-α-glucan.[20]
Biosafety
Botryosphaeran was demonstrated in extensive studies on mice[34][35] and mammalian cell-lines (hamster, rat, human)[36][37][38] that it was not mutagenic (assessed by the micronucleus test),[34][35] nor was it genotoxic as assessed by the Ames test and Comet assay.[36][38]
When administered orally to mice by gavage, botryosphaeran reduced the clastogenic effect of cyclophosphamide-induced micronucleus formation in bone marrow (polychromatic erythrocytes)[34] and peripheral blood (reticulocytes) cells.[34][35]
Using mammalian cell lines: lung fibroblasts (Chinese hamster) and hepatocarcinoma cells (rat), botryosphaeran was confirmed not to be mutagenic nor genotoxic by the micronucleus test and Comet assay procedures.[38] Botryosphaeran exhibited no mutagenicity, and protected cultured human whole blood lymphocytes against DNA damage and cell death induced by bleomycin throughout the cell cycle stage. Botryosphaeran exhibited antigenotoxic activity against damage induced by methyl methanesulfonate, in normal and tumorigenic (Jurkat) human lymphocytes.[36]
The absence of mutagenicity and genotoxicity assessed by the micronucleus, Ames and MTT tests, and the Comet assay, established that botryosphaeran has GRAS status (Generally Recommended As Safe), and is safe for use by humans and animals.
Rheological properties
The rheological properties of botryosphaeran has been described.[39][40]
Botryosphaeran forms a viscous solution when dissolved in water that is stable to heat as occurs during autoclaving (steam sterilization). When an aqueous solution of botryosphaeran is cooled to 5 °C, it forms a strong gel that is firm and transparent.
Biological functions
Botryosphaeran possesses in-vitro free-radical scavenging properties and antioxidant activities.[41]
Botryosphaeran exhibits an in-vivo antioxidant role in the β-cell line INS-1E derived from rat insulinoma (tumor of the pancreas derived from β-cells).[42] Oxidative stress was induced by hydrogen peroxide (H2O2) in the INS-1E cells under high glucose, and botryosphaeran decreased this condition by reducing the production of reactive oxygen species (ROS).[42] Apoptosis increased in the INS-1E cells treated with H2O2 in high glucose conditions, and treatment with botryosphaeran attenuated apoptosis.
Botryosphaeran exerts a chemoprotective effect exhibiting strong antimutagenic (anticlastogenic) activity against the in-vivo DNA-damaging effect of cyclophosphamide in mice.,[35][43] and genotoxic damage by doxorubicin in fibroblasts and hepatocarcinoma cells,[38] bleomycin in human lymphocytes, and methyl methanesulfonate in Jurkat cells.[36]
Botryosphaeran exhibits hypoglycaemic activity (lowering of blood glucose levels) in rats in which diabetes was induced by intramuscular injection of streptozotocin, which selectively damages the pancreatic insulin-secreting β-cells resulting in type-1 diabetes condition.[43]
The cholesterol-lowering effect (hypocholesterolaemia) of β-glucans derived from oat and barley (β-(1→3)(1→4)-linked D-glucans) is well established.[44] Botryosphaeran exhibits hypocholesterolaemic activity lowering total cholesterol and Low Density Lipoprotein (LDL)-cholesterol blood levels in rats preconditioned on hyperlipidaemic diets.[35][45]
In experiments with elderly male knockout LDLr-/-mice (LDLreceptor-deficient mice that show elevated plasma cholesterol levels and develop atherosclerosis), botryosphaeran reduced the plasma glucose levels, improved the lipidic profiles, reduced LDL-cholesterol, and decreased aortic lipid deposition that lowers cardiovascular risks of atherosclerosis.[35]
Treatment of obese rats with botryosphaeran by gavage was effective in ameliorating the comorbidities (diabetes, dyslipidaemia, hepatic steatosis) associated with obesity. Botryosphaeran reduces hepatic steatosis and dyslipidaemia, and glucose intolerance in diet-induced obese rats through activation of AMP-activated protein-kinase (AMPK) and the expression of the Forkhead transcription factor, FOXO3a, in adipose tissue.[45]
Obese rats showed significant increases in weight gain, adipose tissue mass, and adiposity and atherogenic indices, and presented glucose intolerance, insulin resistance, dyslipidaemia, and hepatic steatosis. Botryosphaeran significantly reduces feed intake, weight gains, periepididymal and mesenteric fat, and improves glucose tolerance in obese rats. Botryosphaeran, furthermore, reduces the serum levels of triglyceride and VLDL-cholesterol, and increased HDL-cholesterol and glycogen in liver, and reduces the atherogenic index.[45]
The above data demonstrated the beneficial effects of botryosphaeran in reducing the stimulatory effect of obesity on dyslipidaemia and hepatic steatosis, and can play a potential role in the management of obesity comorbidities.
Studies on human carcinoma cell-lines: Jurkat (lymphocytes)[37] and breast (MCF-7)[46] demonstrated that botryosphaeran manifests anti-cancer activity.
The action by which anticancer activity occurs is still not well understood, but a mechanistic insight on how this may occur in breast cancer MCF-7 cells was advanced in 2015, and involves cell-signaling pathways that suppress tumourigenesis (cell antiproliferation) through apoptosis, necrosis and oxidative stress. Botryosphaeran-induced apoptosis was mediated by AMPK and FOXO3a.[46]
In tumorigenic human lymphocytes (Jurkat cells), botryosphaeran modulates gene expression and regulates cell cycle and the cell cycle checkpoint.[37]
Encapsulation of probiotic bacteria (Lactobacillus casei) in alginate microspheres together with botryosphaeran[47] and mucilages from linseed and okra increases the encapsulation efficiency, and improves the stability of the encapsulated probiotic bacteria during prolonged storage at 5 °C. Gastrointestinal simulation of the microencapsulated Lactobacillus casei cells demonstrated preservation of the viability of the probiotic bacteria against low pH and bile salts.[47] The use of botryosphaeran in microencapsulating probiotic bacteria appears to be another promising application for this β-glucan.
New applications for botryosphaeran have included: biological response modifying activities of derivatized botryosphaerans; treatment of skin conditions (eczema, psoriasis, wound healing); antimicrobial activities; antinociceptive activity; as a matrix for enzyme (laccase) immobilization; as a platform for electrochemical sensors;[48] and as a food additive (nutraceutical).
The biomass resulting from Botryosphaeria rhodina MAMB-05 on producing botryosphaeran by submerged fermentation has been successfully used as a biosorbent to extract metallic species; rare earth elements (lanthanides; La, Sm),[49] and heavy metals (lead; Pb) from industrial effluents.
References
- Dekker, Robert F.H; Barbosa, Aneli M (January 2001). "The effects of aeration and veratryl alcohol on the production of two laccases by the ascomycete Botryosphaeria sp". Enzyme and Microbial Technology. 28 (1): 81–88. doi:10.1016/s0141-0229(00)00274-x. ISSN 0141-0229. PMID 11118601.
- Selbmann, L.; Crognale, S.; Petruccioli, M. (January 2002). "Exopolysaccharide production from Sclerotium glucanicum NRRL 3006 and Botryosphaeria rhodina DABAC-P82 on raw and hydrolysed starchy materials". Letters in Applied Microbiology. 34 (1): 51–55. doi:10.1046/j.1472-765x.2002.01042.x. ISSN 0266-8254. PMID 11849493.
- Barbosa, Aneli M; Steluti, Rosângela M; Dekker, Robert F.H; Cardoso, Marilsa S; Corradi da Silva, M.L (July 2003). "Structural characterization of Botryosphaeran: a (1→3;1→6)-β-d-glucan produced by the ascomyceteous fungus, Botryosphaeria sp". Carbohydrate Research. 338 (16): 1691–1698. doi:10.1016/s0008-6215(03)00240-4. ISSN 0008-6215. PMID 12873424.
- Selbmann, Laura; Stingele, Francesca; Petruccioli, Maurizio (2003-09-01). "Exopolysaccharide production by filamentous fungi: the example of Botryosphaeria rhodina". Antonie van Leeuwenhoek. 84 (2): 135–145. doi:10.1023/A:1025421401536. ISSN 1572-9699. PMID 14533717. S2CID 12997014.
- Weng, Brian Bor-Chun; Lin, Yu-Chih; Hu, Chia-Wen; Kao, Ming-Yuan; Wang, Shih-Hao; Lo, Dan-Yuan; Lai, Tzu-Yuan; Kan, Lou-Sing; Chiou, Robin Yih-Yuan (April 2011). "Toxicological and immunomodulatory assessments of botryosphaeran (β-glucan) produced by Botryosphaeria rhodina RCYU 30101". Food and Chemical Toxicology. 49 (4): 910–916. doi:10.1016/j.fct.2010.10.036. ISSN 0278-6915. PMID 21185904.
- "Botryosphaeria rhodina isolate MAMB 05 18S ribosomal RNA gene, partial sequence; internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence; and 28S ribosomal RNA gene, partial sequence". 2004-04-29.
{{cite journal}}: Cite journal requires|journal=(help) - Saldanha, Roze L.; Garcia, José E.; Dekker, Robert F. H.; Vilas-Boas, Laurival A.; Barbosa, Aneli M. (June 2007). "Genetic diversity among Botryosphaeria isolates and their correlation with cell wall-lytic enzyme production". Brazilian Journal of Microbiology. 38 (2): 259–264. doi:10.1590/s1517-83822007000200013. ISSN 1517-8382.
- Barbosa, A.M.; Dekker, R.F.H.; Hardy, G.E. St (August 1996). "Veratryl alcohol as an inducer of laccase by an ascomycete, Botryosphaeria sp., when screened on the polymeric dye Poly R-478". Letters in Applied Microbiology. 23 (2): 93–96. doi:10.1111/j.1472-765x.1996.tb00038.x. ISSN 0266-8254. S2CID 84782439.
- BARBOSA, A. M. (1995). "In-vivo decolorisation of Poly R-478 as a method for screening ligninolytic microorganisms for use in bioremediation". 4th Pacific Rim Biotechnol. Conf., Melbourne, Australia, Feb 6-9, 1995, Pp. 88-90.
- Dekker, Robert F.H.; Barbosa, Aneli M.; Sargent, Keith (March 2002). "The effect of lignin-related compounds on the growth and production of laccases by the ascomycete, Botryosphaeria sp". Enzyme and Microbial Technology. 30 (3): 374–380. doi:10.1016/s0141-0229(01)00503-8. ISSN 0141-0229.
- Dekker, Robert F.H.; Vasconcelos, Ana-Flora D.; Barbosa, Aneli M.; Giese, Ellen C.; Paccola-Meirelles, Luzia (2001-12-01). "A new role for veratryl alcohol: regulation of synthesis of lignocellulose-degrading enzymes in the ligninolytic ascomyceteous fungus, Botryosphaeria sp.; influence of carbon source". Biotechnology Letters. 23 (24): 1987–1993. doi:10.1023/A:1013742527550. ISSN 1573-6776. S2CID 11468117.
- Steluti, Rosangela M.; Giese, Ellen C.; Piggato, Mariane M.; Sumiya, Andressa F. G.; Covizzi, Luiz G.; Job, Aldo E.; Cardoso, Marilsa S.; De Lourdes Corradi Da Silva, Maria; Dekker, Robert F. H. (December 2004). "Comparison of Botryosphaeran production by the ascomyceteous fungusBotryosphaeria sp., grown on different carbohydrate carbon sources, and their partial structural features". Journal of Basic Microbiology. 44 (6): 480–486. doi:10.1002/jobm.200410415. ISSN 0233-111X. PMID 15558819. S2CID 37841100.
- Giese, Ellen Cristine; Sumiya, Andressa; Borsato, Dioníso; Dekker, Robert; Barbosa, Aneli (2015-04-05). "Evaluation of Fermentative Parameters for the Production of Botryosphaeran (a(1-->3;1-->6)-β-D-glucan) and Mycelial Biomass by Botryosphaeria rhodina MAMB-05". Orbital - the Electronic Journal of Chemistry. 7 (1). doi:10.17807/orbital.v7i1.637. ISSN 1984-6428.
- Silva, Cassiano C.; Dekker, Robert F.H.; Silva, Rui Sérgio S.F.; Silva, Maria de Lourdes Corradi da; Barbosa, Aneli M. (August 2007). "Effect of soybean oil and Tween 80 on the production of botryosphaeran by Botryosphaeria rhodina MAMB-05". Process Biochemistry. 42 (8): 1254–1258. doi:10.1016/j.procbio.2007.05.009. ISSN 1359-5113.
- DELOURDESCORRADIDASILVA, M; IZELI, N; MARTINEZ, P; SILVA, I; CONSTANTINO, C; CARDOSO, M; BARBOSA, A; DEKKER, R; DASILVA, G (2005-07-04). "Purification and structural characterisation of (1→3;1→6)-β--glucans (botryosphaerans) from grown on sucrose and fructose as carbon sources: a comparative study". Carbohydrate Polymers. 61 (1): 10–17. doi:10.1016/j.carbpol.2005.01.002. ISSN 0144-8617.
- Crognale, Silvia; Bruno, Maria; Moresi, Mauro; Petruccioli, Maurizio (July 2007). "Enhanced production of β-glucan from Botryosphaeria rhodina using emulsified media or fan impellers". Enzyme and Microbial Technology. 41 (1–2): 111–120. doi:10.1016/j.enzmictec.2006.12.008. ISSN 0141-0229.
- Giese, Ellen C.; Dekker, Robert F.H.; Scarminio, Ieda S.; Barbosa, Aneli M.; da Silva, Roberto (January 2011). "Comparison of β-1,3-glucanase production by Botryosphaeria rhodina MAMB-05 and Trichoderma harzianum Rifai and its optimization using a statistical mixture-design". Biochemical Engineering Journal. 53 (2): 239–243. doi:10.1016/j.bej.2010.10.013. hdl:11449/72299. ISSN 1369-703X.
- P., Box, George E. (2005). Statistics for experimenters : design, innovation, and discovery. Hunter, J. Stuart, 1923-, Hunter, William Gordon, 1937- (2nd ed.). Hoboken, N.J.: Wiley-Interscience. ISBN 978-0471718130. OCLC 57286064.
{{cite book}}: CS1 maint: multiple names: authors list (link) - E., Bruns, R. (2006). Statistical design--chemometrics. Scarminio, I. S., Barros Neto, B. de. (1st ed.). Amsterdam: Elsevier. ISBN 9780444521811. OCLC 162587290.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Corradi da Silva, Maria de Lourdes; Fukuda, Eliane K.; Vasconcelos, Ana Flora D.; Dekker, Robert F.H.; Matias, Andreza C.; Monteiro, Nilson K.; Cardoso, Marilsa S.; Barbosa, Aneli M.; Silveira, Joana L.M. (March 2008). "Structural characterization of the cell wall d-glucans isolated from the mycelium of Botryosphaeria rhodina MAMB-05". Carbohydrate Research. 343 (4): 793–798. doi:10.1016/j.carres.2007.12.021. ISSN 0008-6215. PMID 18237722.
- Giese, Ellen C.; Covizzi, Luiz G.; Dekker, Robert F.H.; Monteiro, Nilson K.; Corradi da Silva, Maria de Lourdes; Barbosa, Aneli M. (June 2006). "Enzymatic hydrolysis of botryosphaeran and laminarin by β-1,3-glucanases produced by Botryosphaeria rhodina and Trichoderma harzianum Rifai". Process Biochemistry. 41 (6): 1265–1271. doi:10.1016/j.procbio.2005.12.023. ISSN 1359-5113.
- Giese, Ellen Cristine; Monteiro, Alexandre C.; de Melo Barbosa, Aneli; Dekker, Robert F. H.; dos Santos, Osvaldo; de Lourdes Corradi da Silva, Maria; Gomes, Eleni; da Silva, Roberto (January 2009). Evaluation of the β-glucanolytic enzyme complex of Trichoderma harzianum Rifai for the production of gluco-oligosaccharide fragments by enzymatic hydrolysis of 1,3;1,6-β-D-glucans. doi:10.1142/9789812837554_0091. ISBN 9789812837547.
{{cite book}}:|journal=ignored (help) - Bauermeister, Anelize; Amador, Ismael R.; Pretti, Carla P.; Giese, Ellen C.; Oliveira, André L. M.; Alves da Cunha, Mário A.; Rezende, Maria Inês; Dekker, Robert F. H.; Barbosa, Aneli M. (2015-01-05). "β-(1 → 3)-Glucanolytic Yeasts from Brazilian Grape Microbiota: Production and Characterization of β-Glucanolytic Enzymes by Aureobasidium pullulans 1WA1 Cultivated on Fungal Mycelium". Journal of Agricultural and Food Chemistry. 63 (1): 269–278. doi:10.1021/jf504333h. ISSN 0021-8561. PMID 25559084.
- Giese, Ellen C.; Covizzi, Luiz G.; Borsato, Dionísio; Dekker, Robert F.H.; de Lourdes Corradi da Silva, Maria; Barbosa, Aneli M. (December 2005). "Botryosphaeran, a new substrate for the production of β-1,3-glucanases by Botryosphaeria rhodina and Trichoderma harzianum Rifai". Process Biochemistry. 40 (12): 3783–3788. doi:10.1016/j.procbio.2005.04.004. ISSN 1359-5113.
- Giese, Ellen C.; Dekker, Robert F.H.; Barbosa, Aneli M.; da Silva, Roberto (November 2008). "Triple helix conformation of botryosphaeran, a (1→3;1→6)-β-d-glucan produced by Botryosphaeria rhodina MAMB-05". Carbohydrate Polymers. 74 (4): 953–956. doi:10.1016/j.carbpol.2008.04.038. ISSN 0144-8617.
- Bohn, John A.; BeMiller, James N. (January 1995). "(1→3)-β-d-Glucans as biological response modifiers: a review of structure-functional activity relationships". Carbohydrate Polymers. 28 (1): 3–14. doi:10.1016/0144-8617(95)00076-3. ISSN 0144-8617.
- Leung, M.Y.K.; Liu, C.; Koon, J.C.M.; Fung, K.P. (June 2006). "Polysaccharide biological response modifiers". Immunology Letters. 105 (2): 101–114. doi:10.1016/j.imlet.2006.01.009. ISSN 0165-2478. PMID 16554097.
- Wang, Qiang; Sheng, Xiaojing; Shi, Aimin; Hu, Hui; Yang, Ying; Liu, Li; Fei, Ling; Liu, Hongzhi (2017-02-09). "β-Glucans: Relationships between Modification, Conformation and Functional Activities". Molecules. 22 (2): 257. doi:10.3390/molecules22020257. ISSN 1420-3049. PMC 6155770. PMID 28208790.
- Mendes, Simone Ferreira; Santos, Osvaldo dos; Barbosa, Aneli M.; Vasconcelos, Ana Flora D.; Aranda-Selverio, Gabriel; Monteiro, Nilson K.; Dekker, Robert F.H.; Pereira, Mariana Sá; Tovar, Ana Maria F. (October 2009). "Sulfonation and anticoagulant activity of botryosphaeran from Botryosphaeria rhodina MAMB-05 grown on fructose". International Journal of Biological Macromolecules. 45 (3): 305–309. doi:10.1016/j.ijbiomac.2009.06.004. ISSN 0141-8130. PMID 19549543.
- Brandi, Jamile (2011-10-28). "Chemical Modification of Botryosphaeran: Structural Characterization and Anticoagulant Activity of a Water-Soluble Sulfonated (1→3)(1→6)-β-D-Glucan". Journal of Microbiology and Biotechnology. 21 (10): 1036–1042. doi:10.4014/jmb.1105.05020. ISSN 1017-7825. PMID 22031027.
- Vasconcelos, Ana Flora D.; Monteiro, Nilson K.; Dekker, Robert F.H.; Barbosa, Aneli M.; Carbonero, Elaine R.; Silveira, Joana L.M.; Sassaki, Guilherme L.; da Silva, Roberto; de Lourdes Corradi da Silva, Maria (September 2008). "Three exopolysaccharides of the β-(1→6)-d-glucan type and a β-(1→3;1→6)-d-glucan produced by strains of Botryosphaeria rhodina isolated from rotting tropical fruit". Carbohydrate Research. 343 (14): 2481–2485. doi:10.1016/j.carres.2008.06.013. ISSN 0008-6215. PMID 18639868.
- Alves da Cunha, Mário A.; Turmina, Janaína A.; Ivanov, Raphael C.; Barroso, Roney R.; Marques, Patrícia T.; Fonseca, Eveline A. I.; Fortes, Zuleica B.; Dekker, Robert F. H.; Khaper, Neelam (2012-03-08). "Lasiodiplodan, an exocellular (1→6)-β-d-glucan from Lasiodiplodia theobromae MMPI: production on glucose, fermentation kinetics, rheology and anti-proliferative activity". Journal of Industrial Microbiology & Biotechnology. 39 (8): 1179–1188. doi:10.1007/s10295-012-1112-2. ISSN 1367-5435. PMID 22399240. S2CID 15375855.
- Oliveira, Kassandra S.M.; Di Bastiani, Mirela; Cordeiro, Lucimara M.C.; Costa, Mírian F.; Toledo, Karina A.; Iacomini, Marcello; Babosa, Aneli M.; Dekker, Robert F.H.; Nascimento, Valéria M.G. (November 2015). "(1→6)- and (1→3)(1→6)-β-glucans from Lasiodiplodia theobromae MMBJ: Structural characterization and pro-inflammatory activity". Carbohydrate Polymers. 133: 539–546. doi:10.1016/j.carbpol.2015.07.060. ISSN 0144-8617. PMID 26344312.
- Miranda, Carolina C.B.O.; Dekker, Robert F.H.; Serpeloni, Juliana M.; Fonseca, Eveline A.I.; Cólus, Ilce M.S.; Barbosa, Aneli M. (March 2008). "Anticlastogenic activity exhibited by botryosphaeran, a new exopolysaccharide produced by Botryosphaeria rhodina MAMB-05". International Journal of Biological Macromolecules. 42 (2): 172–177. doi:10.1016/j.ijbiomac.2007.10.010. ISSN 0141-8130. PMID 18022685.
- Silva-Sena, Geralda Gillian; Malini, Maressa; Delarmelina, Juliana Macedo; Dutra, Jean Carlos Vencioneck; Gervásio, Suiany Vitorino; Leal, Marcos André Soares; Costa Pereira, Thiago de Melo; Barbosa-Dekker, Aneli M.; Dekker, Robert F.H. (February 2018). "In vivo antimutagenic and antiatherogenic effects of the (1 → 3)(1 → 6)-β-d- glucan botryosphaeran". Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 826: 6–14. doi:10.1016/j.mrgentox.2017.12.008. ISSN 1383-5718. PMID 29412871.
- Kerche-Silva, Leandra E.; Cólus, Ilce M.S.; Malini, Maressa; Mori, Mateus Prates; Dekker, Robert F.H.; Barbosa-Dekker, Aneli M. (February 2017). "In vitro protective effects of botryosphaeran, a (1 → 3;1 → 6)-β- d -glucan, against mutagens in normal and tumor rodent cells". Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 814: 29–36. doi:10.1016/j.mrgentox.2016.12.003. ISSN 1383-5718. PMID 28137365.
- Malini, M.; Camargo, M.S.; Hernandes, L.C.; Vargas-Rechia, C.G.; Varanda, E.A.; Barbosa, A.M.; Dekker, R.F.H.; Matsumoto, S.T.; Antunes, L.M.G. (October 2016). "Chemopreventive effect and lack of genotoxicity and mutagenicity of the exopolysaccharide botryosphaeran on human lymphocytes". Toxicology in Vitro. 36: 18–25. doi:10.1016/j.tiv.2016.06.008. hdl:11449/161952. ISSN 0887-2333. PMID 27387458.
- Malini, Maressa; Souza, Marilesia Ferreira de; Oliveira, Marcelo Tempesta de; Antunes, Lusânia Maria Greggi; Figueiredo, Suely Gomes de; Barbosa, Aneli M.; Dekker, Robert F.H.; Cólus, Ilce Mara de Syllos (June 2015). "Modulation of gene expression and cell cycle by botryosphaeran, a (1→3)(1→6)-β-d-glucan in human lymphocytes". International Journal of Biological Macromolecules. 77: 214–221. doi:10.1016/j.ijbiomac.2015.03.010. ISSN 0141-8130. PMID 25795388.
- BONGIOVANI, Raphael A. M. (2009-04-09). "Nota Científica: Caracterização reológica dos botriosferanas produzidos pelo Botryosphaeria rhodina MAMB-05 em glucose, sacarose e frutose como fontes de carbono". Brazilian Journal of Food Technology. 12 (1): 53–59. doi:10.4260/bjft2009800900008. ISSN 1981-6723.
- Fonseca, Paulo R.M.S.; Dekker, Robert F.H.; Barbosa, Aneli M.; Silveira, Joana L.M.; Vasconcelos, Ana F.D.; Monteiro, Nilson K.; Aranda-Selverio, Gabriel; Da Silva, Maria de Lourdes Corradi (2011-09-02). "Thermal and Rheological Properties of a Family of Botryosphaerans Produced by Botryosphaeria rhodina MAMB-05". Molecules. 16 (9): 7488–7501. doi:10.3390/molecules16097488. ISSN 1420-3049. PMC 6264532. PMID 21892127.
- Giese, Ellen C.; Gascon, Jacob; Anzelmo, Gianluca; Barbosa, Aneli M.; da Cunha, Mário A. Alves; Dekker, Robert F.H. (January 2015). "Free-radical scavenging properties and antioxidant activities of botryosphaeran and some other β-D-glucans". International Journal of Biological Macromolecules. 72: 125–130. doi:10.1016/j.ijbiomac.2014.07.046. ISSN 0141-8130. PMID 25128096.
- Kazak, Hande (2014). Biological activities of bacterial levan, and three fungal β-glucans, botryosphaeran and lasiodiplodan, under high glucose condition in the pancreatic β-cell line INS-1E. Narosa Publishing House, New Delhi, India. pp. Vol. 7, Ch. 8, p. 105–115. doi:10.13140/2.1.1799.2649. ISBN 978-81-8487-214-9.
- Miranda-Nantes, Carolina C. B. O.; Fonseca, Eveline A. I.; Zaia, Cassia T. B. V.; Dekker, Robert F. H.; Khaper, Neelam; Castro, Inar A.; Barbosa, Aneli M. (September 2011). "Hypoglycemic and Hypocholesterolemic Effects of Botryosphaeran from Botryosphaeria rhodina MAMB-05 in Diabetes-Induced and Hyperlipidemia Conditions in Rats". Mycobiology. 39 (3): 187–193. doi:10.5941/myco.2011.39.3.187. ISSN 1229-8093. PMC 3385115. PMID 22783102.
- Daou, Cheickna; Zhang, Hui (2012-06-12). "Oat Beta-Glucan: Its Role in Health Promotion and Prevention of Diseases". Comprehensive Reviews in Food Science and Food Safety. 11 (4): 355–365. doi:10.1111/j.1541-4337.2012.00189.x. ISSN 1541-4337.
- Silva, Amadeu Z.; Costa, Felipe P.L.; Souza, Ingrid L.; Ribeiro, Mariana C.; Giordani, Morenna Alana; Queiroz, Diogo A.; Luvizotto, Renata A.M.; Nascimento, André F.; Bomfim, Gisele F. (October 2018). "Botryosphaeran reduces obesity, hepatic steatosis, dyslipidaemia, insulin resistance and glucose intolerance in diet-induced obese rats". Life Sciences. 211: 147–156. doi:10.1016/j.lfs.2018.09.027. ISSN 0024-3205. PMID 30227131. S2CID 52293952.
- Queiroz, Eveline A.I.F.; Fortes, Zuleica B.; da Cunha, Mário A.A.; Barbosa, Aneli M.; Khaper, Neelam; Dekker, Robert F.H. (October 2015). "Antiproliferative and pro-apoptotic effects of three fungal exocellular β-glucans in MCF-7 breast cancer cells is mediated by oxidative stress, AMP-activated protein kinase (AMPK) and the Forkhead transcription factor, FOXO3a". The International Journal of Biochemistry & Cell Biology. 67: 14–24. doi:10.1016/j.biocel.2015.08.003. ISSN 1357-2725. PMID 26255117.
- Rodrigues, Fábio J.; Omura, Michele H.; Cedran, Marina F.; Dekker, Robert F. H.; Barbosa-Dekker, Aneli M.; Garcia, Sandra (2017-07-04). "Effect of natural polymers on the survival of Lactobacillus casei encapsulated in alginate microspheres". Journal of Microencapsulation. 34 (5): 431–439. doi:10.1080/02652048.2017.1343872. ISSN 0265-2048. PMID 28618877. S2CID 41482357.
- Eisele, Ana Paula Pires; Valezi, Camila Farinha; Mazziero, Tatiane; Dekker, Robert F.H.; Barbosa-Dekker, Aneli M.; Sartori, Elen Romão (May 2019). "Layering of a film of carboxymethyl-botryosphaeran onto carbon black as a novel sensitive electrochemical platform on glassy carbon electrodes for the improvement in the simultaneous determination of phenolic compounds". Sensors and Actuators B: Chemical. 287: 18–26. doi:10.1016/j.snb.2019.02.004. ISSN 0925-4005. S2CID 104322350.
- Giese, Ellen C.; Dekker, Robert F.H.; Barbosa-Dekker, Aneli M. (2019-02-09). "Biosorption of lanthanum and samarium by viable and autoclaved mycelium of Botryosphaeria rhodina MAMB-05". Biotechnology Progress. 35 (3): e2783. doi:10.1002/btpr.2783. ISSN 8756-7938. PMID 30738002. S2CID 73427692.