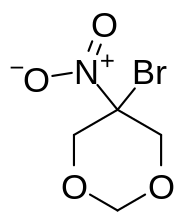
Bronidox
Bronidox, or 5-bromo-5-nitro-1,3-dioxane, is an antimicrobial chemical compound.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
5-Bromo-5-nitro-1,3-dioxane | |
| Other names
5-Bromo-5-nitro-m-dioxane | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.045.441 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H6BrNO4 | |
| Molar mass | 211.999 g·mol−1 |
| Appearance | White crystalline powder |
| Melting point | 60 °C (140 °F; 333 K)[1] 58.5−62 °C[2] |
| insoluble | |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H302, H314, H315, H317, H410 | |
| P260, P261, P264, P270, P272, P273, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P330, P332+P313, P333+P313, P362, P363, P391, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
590 mg/kg (mouse, oral) 455 mg/kg (rat, oral) 31 mg/kg (rat, ipr.) 2500 µg (mouse, skin) 2500 µg (rat, skin) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Bronidox causes inhibition of enzyme activity in bacteria.[3]
Bronidox is corrosive to metals.[4]
Uses
- Bactericide
- Fungicide, effective against yeast and other fungi
- It is used in immunology for preserving antibodies and antisera in 0.1 - 0.5% concentration. It is used as preservative to avoid use of sodium azide.
- Stabilizer
- Surfactant
- Used in cosmetics since the mid-1970s as preservative for shampoos, foam bath, etc. Maximum concentration is 0.1%.
- Some users do not recommend use in preparations destined for in vivo or tissue culture work[5]
See also
References
- 5-BROMO-5-NITRO-1,3-DIOXANE Archived March 3, 2016, at the Wayback Machine, chemicalland21.com
- "Bronidox, Product Information Sheet" (PDF). Archived from the original (PDF) on September 29, 2007. Retrieved March 19, 2006.
- Ghannoum M, Thomson M, Bowman W, Al-Khalil S (1986). "Mode of action of the antimicrobial compound 5-bromo-5-nitro-1,3-dioxane (bronidox)". Folia Microbiol. (Praha). 31 (1): 19–31. doi:10.1007/BF02928676. PMID 3082729. S2CID 23920602.
- http://www.products.cognis.com/COGNIS/prodleaf.nsf/(SBU_Catalog)/FFAD30C61B67EFEB41256B4100427DD1/$File/BRONIDOX_r_L_5_E.pdf
- Archived December 21, 2004, at the Wayback Machine
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.