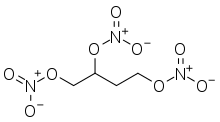
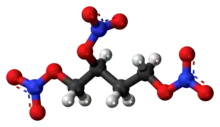
1,2,4-Butanetriol trinitrate
1,2,4-Butanetriol trinitrate (BTTN), also called butanetriol trinitrate, is an important military propellant. It is a colorless to brown explosive liquid.[1]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butane-1,2,4-triyl trinitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.026.998 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H7N3O9 | |
| Molar mass | 241.11 g/mol |
| Density | 1.52 g/cm3 |
| Melting point | 25 °C |
| Boiling point | 230 °C (446 °F; 503 K) (explosion temperature) |
| Hazards | |
| GHS labelling: | |
    | |
| Danger | |
| H200, H300, H310, H330, H373, H411 | |
| P201, P202, P260, P262, P264, P270, P271, P273, P280, P281, P284, P301+P310, P302+P350, P304+P340, P310, P314, P320, P321, P322, P330, P361, P363, P372, P373, P380, P391, P401, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
BTTN is used as a propellant in virtually all single-stage missiles used by the United States, including the Hellfire.[2] It is less volatile, less sensitive to shock, and more thermally stable than nitroglycerine,[3] for which it is a promising replacement.[4]
BTTN as a propellant is often used in a mixture with nitroglycerin.[3] The mixture can be made by co-nitration of butanetriol and glycerol.[5] BTTN is also used as a plasticizer in some nitrocellulose-based propellants.[6]
BTTN is manufactured by nitration of 1,2,4-butanetriol.[7] Biotechnological manufacture of butanetriol is under intensive research.[8]
References
- Pisacane, Frank J. (1982). 1,2,4-Butanetriol: Analysis and Synthesis. PN.
- "Bacteria help make missile fuel". 2004-02-02. Retrieved 2018-11-02.
- Varghese, T. L.; Krishnamurthy, V. N. (2017-01-03). The Chemistry and Technology of Solid Rocket Propellants (A Treatise on Solid Propellants). Allied Publishers. p. 187. ISBN 978-93-85926-33-4.
- Bhowmik, D.; Sadavarte, V.S.; Pande, S.M.; Saraswat, B.S. (2015). "An Energetic Binder for the Formulation of Advanced Solid Rocket Propellants". Central European Journal of Energetic Materials. 12 (1): 147.
- Farncomb, Robert E.; Carr, Walter A. (1987-07-06). "Patent application: Co-Nitration of 1,2,4-Butanetriol and Glycerin". Archived from the original on June 1, 2022.
- Sutton, George P.; Biblarz, Oscar (2016-11-30). Rocket Propulsion Elements. John Wiley & Sons. ISBN 978-1-118-75391-0.
- Gouranlou, Farideh; Kohsary, Iraj (2010-06-01). "Synthesis and Characterization of 1,2,4-Butanetrioltrinitrate". Asian Journal of Chemistry. 22: 4221–4228.
- Cao, Yujin; Niu, Wei; Guo, Jiantao; Xian, Mo; Liu, Huizhou (2015-12-16). "Biotechnological production of 1,2,4-butanetriol: An efficient process to synthesize energetic material precursor from renewable biomass". Scientific Reports. 5 (1): 18149. Bibcode:2015NatSR...518149C. doi:10.1038/srep18149. ISSN 2045-2322. PMC 4680960. PMID 26670289.