Glabridin
Glabridin is a chemical compound that is found in the root extract of licorice (Glycyrrhiza glabra).[2] Glabridin is an isoflavane, a type of isoflavonoid. This product is part of a larger family of plant-derived molecules, the natural phenols. Glabridin effectively inhibits platelet activation, so it might become therapeutic agent for thromboembolic disorders.[3]
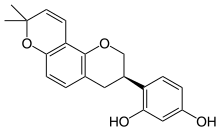 | |
| Names | |
|---|---|
| IUPAC name
(3R)-6′′,6′′-Dimethyl-6′′H-pyrano[2′′,3′′:7,8]isoflavan-2′,4′-diol | |
| Systematic IUPAC name
4-[(3R)-8,8-Dimethyl-3,4-dihydro-2H,8H-(benzo[1,2-b:3,4-b′]dipyran)-3-yl]benzene-1,3-diol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.126.141 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H20O4 | |
| Molar mass | 324.376 g·mol−1 |
| Appearance | Yellowish-brown powder |
| Melting point | 238–240 °C (460–464 °F; 511–513 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It is used as an ingredient in cosmetics and is listed in International Nomenclature of Cosmetic Ingredients (INCI).
Glabridin is yellowish-brown powder. It is insoluble in water, but soluble in organic solvents such as propylene glycol.
See also
References
- SciFinder Record for CAS#59870-68-7
- Kinoshita T, Kajiyama K, Hiraga Y, Takahashi K, Tamura Y, Mizutani K (1996). "Isoflavan derivatives from Glycyrrhiza glabra (licorice)". Heterocycles. 43 (3): 581–588.
- Chung CL, Chen JH, Huang WC, Sheu JR, Hsia CW, Jayakumar T, Hsia CH, Chiou KR, Hou SM (September 2022). "Glabridin, a Bioactive Flavonoid from Licorice, Effectively Inhibits Platelet Activation in Humans and Mice". International Journal of Molecular Sciences. 23 (19). doi:10.3390/ijms231911372. PMC 9570097.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.