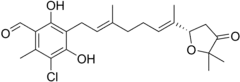
Ascofuranone
Ascofuranone is an antibiotic produced by various ascomycete fungi including Acremonium sclerotigenum[1] that inhibits the Trypanosoma brucei alternative oxidase and is a lead compound in efforts to produce other drugs targeting this enzyme for the treatment of sleeping sickness.[2] The compound is effective both in vitro cell culture and in infections in mice.[3]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-Chloro-5-{(2E,6E)-7-[(2S)-5,5-dimethyl-4-oxooxolan-2-yl]-3-methylocta-2,6-dien-1-yl}-4,6-dihydroxy-2-methylbenzaldehyde | |
| Other names
Ascofuranon | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C23H29ClO5 | |
| Molar mass | 420.93 g·mol−1 |
| Density | 1.207 g/mL |
| Boiling point | 581.2 °C (1,078.2 °F; 854.4 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ascofuranone has also been reported to have anti-tumor activity,[4] and modulate the immune system.[5]
Biosynthesis
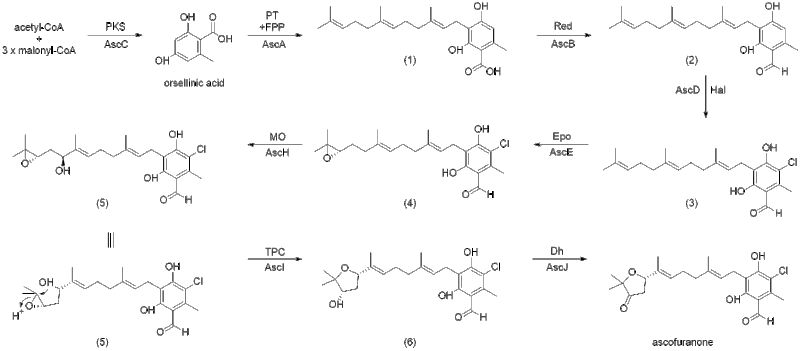
The proposed biosynthesis of ascofuranone was reported by Kita et al., as well as by Abe et al.[6][7] The prenylation of orsellinic acid, followed by terminal cyclization through epoxidation is how ascofuranone can be synthesized. Compound (1), ilicicolinic acid B, was found to be produced from polyketide synthase (PKS) StBA and that AscCABD are responsible for the biosynthesis of ilicicolin A (3). Ilicicolin B (2) was found to be produced by expressing AscC (polyketide synthase) which is then followed by expression of AscA (prenyltransferase). AscD (flavin-dependent halogenase, flavin binding enzyme) is able to catalyze the chlorination of ilicicolinic acid B (2) to yield ilicicolin A (3).
Expodiation of (3) by AscE (P450 monooxygenase) leads to the formation of ilicicolin A epoxide (4). Ilicicolin A epoxide can then be hydroxylated by AscH at C-16 (P450 monooygenase) to yield intermediate (5) which can then be cyclized by AscI (eight-transmembrane protein, TPC) into ascofuranol (6), specifically through 6-endo-tet cyclization. Finally, ascofuranol (6) can be oxidized by AscJ (NAD(P)-dependent alcohol dehydrogenase) leading to the formation of ascofuranone.
References
- Hijikawa Y, Matsuzaki M, Suzuki S, Inaoka DK, Tatsumi R, Kido Y, Kita K (March 2017). "Re-identification of the ascofuranone-producing fungus Ascochyta viciae as Acremonium sclerotigenum". The Journal of Antibiotics. 70 (3): 304–307. doi:10.1038/ja.2016.132. PMID 27804952. S2CID 11725030.
- Minagawa N, Yabu Y, Kita K, Nagai K, Ohta N, Meguro K, et al. (February 1997). "An antibiotic, ascofuranone, specifically inhibits respiration and in vitro growth of long slender bloodstream forms of Trypanosoma brucei brucei". Molecular and Biochemical Parasitology. 84 (2): 271–280. doi:10.1016/S0166-6851(96)02797-1. PMID 9084049.
- Yabu Y, Yoshida A, Suzuki T, Nihei C, Kawai K, Minagawa N, et al. (June 2003). "The efficacy of ascofuranone in a consecutive treatment on Trypanosoma brucei brucei in mice". Parasitology International. 52 (2): 155–164. doi:10.1016/S1383-5769(03)00012-6. PMID 12798927.
- Magae J, Hayasaki J, Matsuda Y, Hotta M, Hosokawa T, Suzuki S, et al. (July 1988). "Antitumor and antimetastatic activity of an antibiotic, ascofuranone, and activation of phagocytes". The Journal of Antibiotics. 41 (7): 959–965. doi:10.7164/antibiotics.41.959. PMID 3417568.
- Magae J, Suzuki S, Nagai K, Yamasaki M, Ando K, Tamura G (March 1986). "In vitro effects of an antitumor antibiotic, ascofuranone, on the murine immune system". Cancer Research. 46 (3): 1073–1078. PMID 3080231.
- Araki Y, Awakawa T, Matsuzaki M, Cho R, Matsuda Y, Hoshino S, et al. (April 2019). "Complete biosynthetic pathways of ascofuranone and ascochlorin in Acremonium egyptiacum". Proceedings of the National Academy of Sciences of the United States of America. 116 (17): 8269–8274. doi:10.1073/pnas.1819254116. PMC 6486709. PMID 30952781.
- Awakawa T, Abe I (June 2021). "Reconstitution of Polyketide-Derived Meroterpenoid Biosynthetic Pathway in Aspergillus oryzae". Journal of Fungi. 7 (6): 486. doi:10.3390/jof7060486. PMC 8235479. PMID 34208768.