Apiin
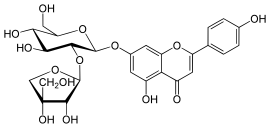
Apiin is a natural flavonoid, a diglycoside of the flavone apigenin found in the winter-hardy plants parsley[1] and celery,[2] and in banana leaf.[3] The glycoside moiety at carbon-7 of apigenin, O-β-D-apiofuranosyl(→)2-β-D-glucosyl, is carried by several other flavones in parsley plant and seed.[4] The sugar apiose possibly play a role in winter hardiness of celery, duckweed and parsley.[5]
 | |
| Names | |
|---|---|
| IUPAC name
4′,5-Dihydroxy-7-[3-C-(hydroxymethyl)-β-D-erythrofuranosyl-(1→2)-β-D-glucopyranosyloxy]flavone | |
| Systematic IUPAC name
7-{[(2S,3R,4S,5S,6R)-2-{[(2S,3R,4R)-3,4-Dihydroxy-4-(hydroxymethyl)oxolan-2-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-5-hydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
Apioside Apigenin-7-apioglucoside Apigenin-7-O-apioglucoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.043.421 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C26H28O14 | |
| Molar mass | 564.496 g·mol−1 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
See also
References
- H. Meyer; A. Bolarinwa; G. Wolfram; J. Linseisen (2006). "Bioavailability of Apigenin from Apiin-Rich Parsley in Humans". Ann Nutr Metab. 50 (3): 167–172. doi:10.1159/000090736. PMID 16407641. S2CID 8223136.
- S. R. Gupta (1952). "A study of apiin from the parsley seeds and plant". Proceedings of the Indian Academy of Sciences, Section A. 35 (5). doi:10.1007/BF03172503. S2CID 91953908.
- Sayadi, Khali; Akbarzadeh, Fatemeh; Pourmardan, Vahid; Saravani-Aval, Mehdi; Sayadi, Jalis; Chauhan, Narendra Pal Singh; Sargazi, Ghasem (2021). "Methods of green synthesis of Au NCs with emphasis on their morphology: A mini-review". Heliyon. Cell Press. 7 (6): e07250. doi:10.1016/j.heliyon.2021.e07250. ISSN 2405-8440. PMC 8220187. PMID 34189304.
- page 245 "Methods in plant biochemistry" volume 2: "Carbohydrates", ISBN 0080984207
- page 136 "Advances in Carbohydrate Chemistry and Biochemistry", Volume 31, ISBN 0080562906
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.