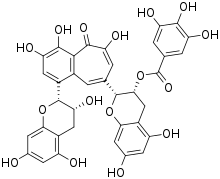
Theaflavin-3-gallate
Theaflavin-3-gallate is a theaflavin derivative. It can be found in abundance in black tea and is produced during fermentation.[1][2][3] It has been studied as a cancer-fighting chemical when combined with cisplatin against ovarian cancer cells.[4][5] [6] Consuming large amounts of black tea has been reported to reduce the effects of aging in female populations.[7][8]
 | |
| Names | |
|---|---|
| Systematic IUPAC name
(2R,3R)-5,7-Dihydroxy-2-{3,4,6-trihydroxy-5-oxo-8-[(2R,3R)-3,5,7-trihydroxy-3,4-dihydro-2H-1-benzopyran-2-yl]-5H-benzo[7]annulen-2-yl}-3,4-dihydro-2H-1-benzopyran-3-yl 3,4,5-trihydroxybenzoate | |
| Other names
Theaflavin-3-monogallate; Theaflavin monogallate A; Theaflavin 2A; TFMG | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C36H28O16 | |
| Molar mass | 716.604 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
See also
References
- X. Hu, Z. Ping, M. Gan et al., “Theaflavin-3,3′-digallate represses osteoclastogenesis and prevents wear debris-induced osteolysis via suppression of ERK pathway,” Acta Biomaterialia 48 (2017): 479–488.
- Shan Z, Nisar M, Li M, Zhang C, Wan C. Theaflavin Chemistry and Its Health Benefits Oxidative Medicine and Cellular Longevity. 2021 Jan;2021. PMCID: PMC8601833.
- Mario A. Vermeer, Theo P. J. Mulder and Henri O. F. Molhuizen (2008). "Theaflavins from Black Tea, Especially Theaflavin-3-gallate, Reduce the Incorporation of Cholesterol into Mixed Micelles". J. Agric. Food Chem. 56 (24): 12031–12036. doi:10.1021/jf8022035. PMID 19049290.
- Shan Z, Nisar M, Li M, Zhang C, Wan C. Theaflavin Chemistry and Its Health Benefits Oxidative Medicine and Cellular Longevity. 2021 Jan;2021. PMCID: PMC8601833.
- Y. Gao, J. Yin, Y. Tu, and Y. Chen, “Theaflavin-3, 3′-digallate suppresses human ovarian carcinoma OVCAR-3 cells by regulating the checkpoint kinase 2 and p27 kip1 pathways,” Molecules 24 (4): 673, 2019
- H. Pan, E. Kim, G. Rankin, Y. Rojanasakul, Y. Tu, and Y. Chen, “Theaflavin-3, 3′-Digallate enhances the inhibitory effect of cisplatin by regulating the copper transporter 1 and glutathione in human ovarian cancer cells,” International Journal of Molecular Sciences 19 (1): 117, 2018.
- Shan Z, Nisar M, Li M, Zhang C, Wan C., "Theaflavin Chemistry and Its Health Benefits", Oxidative Medicine and Cellular Longevity 2021 Jan; 2021. PMCID: PMC8601833.
- Y. Oka, S. Iwai, H. Amano et al., “Tea polyphenols inhibit rat osteoclast formation and differentiation,” Journal of Pharmacological Sciences 118 (1): 55–64, 2012.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.