Cocoa butter
Cocoa butter, also called theobroma oil, is a pale-yellow, edible fat extracted from the cocoa bean (Theobroma cacao). It is used to make chocolate, as well as some ointments, toiletries, and pharmaceuticals.[2] Cocoa butter has a cocoa flavor and aroma. Its melting point is slightly below human body temperature. It is an essential major ingredient of chocolate and related confectionary products.
 Raw cocoa butter | |
| Fat composition | |
|---|---|
| Saturated fats | |
| Total saturated | 57–64%: stearic acid (24–37%), palmitic acid (24–30%), myristic acid, (0–4%), arachidic acid (1%), lauric acid (0–1%) |
| Unsaturated fats | |
| Total unsaturated | 36–43% |
| Monounsaturated | 29–43%: oleic acid (29–38%), palmitoleic acid (0–2%) |
| Polyunsaturated | 0–5%: linoleic acid (0–4%), α-Linolenic acid (0–1%) |
| Properties | |
| Food energy per 100 g (3.5 oz) | 3,699 kilojoules (884 kcal)[1] |
| Melting point | 34.1 °C (93.4 °F), 35–36.5 °C (95.0–97.7 °F) |
| Solidity at 20 °C (68 °F) | solid |
| Refractive index | 1.44556–1.44573 |
| Iodine value | 32.11–35.12, 35.575 |
| Acid value | 1.68 |
| Saponification value | 191.214, 192.88–196.29 |
Extraction and composition
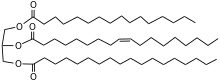
For use in chocolate manufacture, the cocoa beans are first fermented and then dried. The beans are then roasted and separated from their hulls to produce cocoa nibs.[3] About 54–58% of the cocoa nibs is cocoa butter. The cocoa nibs are ground to form cocoa mass, also known as cocoa liquor or chocolate liquor. Chocolate liquor is pressed to separate the cocoa butter from the non-fat cocoa solids.[4] Cocoa butter is sometimes deodorized to remove strong or undesirable tastes.[5]
Cocoa butter contains a high proportion of saturated fats also with the monounsaturated oleic acid in each triglyceride. The predominant triglycerides are POS, SOS, POP, where P = palmitic, O = oleic, and S = stearic acid residues.[6][7][8][9] Cocoa butter, unlike non-fat cocoa solids, contains only traces of caffeine and theobromine.[10]
| Fatty acid | Percentage |
|---|---|
| Arachidic acid (C20:0) | 1.0% |
| Linoleic acid (C18:2) | 3.2% |
| Oleic acid (C18:1) | 34.5% |
| Palmitic acid (C16:0) | 26.0% |
| Palmitoleic acid (C16:1) | 0.3% |
| Stearic acid (C18:0) | 34.5% |
| Other Fatty Acids | 0.5% |
Adulterants and substitutes
Some food manufacturers substitute less expensive materials in place of cocoa butter. Several analytical methods exist for testing for diluted cocoa butter. Adulterated cocoa butter is indicated by its lighter color and its diminished fluorescence upon ultraviolet illumination. Unlike cocoa butter, adulterated fat tends to smear and have a higher non-saponifiable content.[12]
Owing to the high cost of cocoa butter,[13][14] substitutes have been designed to use as alternatives. In the United States, 100% cocoa butter must be used for the product to be called chocolate. The EU requires that alternative fats not exceed 5% of the total fat content.[11]
Substitutes include: coconut, palm,[11] soybean, rapeseed, cottonseed and illipe oils; and shea butter, mango kernel fat[15] and a mixture of mango kernel fat and palm oil,[16] and PGPR.
Uses
Cocoa butter is a major ingredient in practically all types of chocolates (white chocolate, milk chocolate, and dark chocolate). This application continues to dominate consumption of cocoa butter.
Pharmaceutical companies use cocoa butter extensively. As a nontoxic solid at room temperature that melts at body temperature, it is considered an ideal base for medicinal suppositories.[17]
Personal care
For a fat melting around body temperature, cocoa has good stability. This quality, coupled with natural antioxidants, prevents rancidity – giving it a storage life of two to five years.[18] The velvety texture, pleasant fragrance and emollient properties of cocoa butter have made it a popular ingredient in products for the skin, such as soaps and lotions.
Physical properties
.jpg.webp)
Cocoa butter typically has a melting point of around 34–38 °C (93–100 °F), so chocolate is solid at room temperature but readily melts once inside the mouth. Cocoa butter displays polymorphism, having different crystalline forms with different melting points. Conventionally the assignment of cocoa butter crystalline forms uses the nomenclature of Wille and Lutton[19] with forms I, II, III, IV, V and VI having melting points 17.3, 23.3, 25.5, 27.5, 33.8, and 36.3 °C (63.1, 73.9, 77.9, 81.5, 92.8, and 97.3 °F), respectively. The production of chocolate aims to crystallise the chocolate so that the cocoa butter is predominantly in form V, which is the most stable form that can be obtained from melted cocoa butter. (Form VI either develops in solid cocoa butter after long storage, or is obtained by crystallisation from solvents). A uniform form V crystal structure will result in smooth texture, sheen, and snap. This structure is obtained by chocolate tempering. Melting the cocoa butter in chocolate and then allowing it to solidify without tempering leads to the formation of unstable polymorphic forms of cocoa butter. This can easily happen when chocolate bars are allowed to melt in a hot room and leads to the formation of white patches on the surface of the chocolate called fat bloom or chocolate bloom.[3]
Further reading
- Jahurul, M. H. A.; Zaidul, I. S. M.; Norulaini, N. A. N.; Sahena, F.; Jinap, S.; Azmir, J.; Sharif, K. M.; Omar, A. K. Mohd (1 August 2013). "Cocoa butter fats and possibilities of substitution in food products concerning cocoa varieties, alternative sources, extraction methods, composition, and characteristics". Journal of Food Engineering. SI: Extraction and Encapsulation. 117 (4): 467–476. doi:10.1016/j.jfoodeng.2012.09.024. ISSN 0260-8774.
References
- "Cocoa butter amounts converter". Convert-to.com. 15 August 2011. Retrieved 3 November 2016.
- "Cocoa butter". Encyclopædia Britannica. July 1998. Retrieved 10 September 2007.
- Industrial Chocolate Manufacture and Use, 4th Edition, ed S.T. Beckett, Chapter 12, G. Talbot
- "Cocoa butter pressing". The Grenada Chocolate Company. Archived from the original on 6 October 2007.
- The Nibble. "The World's Best White Chocolate Page 3: Percent Cacao & Cocoa Butter". Retrieved 3 March 2009.
- Lonchampt, P.; Hartel Richard, W. (2004). "Fat bloom in chocolate and compound coatings". European Journal of Lipid Science and Technology. 106 (4): 241–274. doi:10.1002/ejlt.200400938.
- "Composition of the Cocoa Bean". Hershey Center for Health & Nutrition. Retrieved 20 November 2012.
- Liendo, Rigel; Padilla, Fanny C.; Quintana, Agricia (November 1997). "Characterization of cocoa butter extracted from Criollo cultivars of Theobroma cacao L.". Food Research International. 30 (9): 727–731. doi:10.1016/S0963-9969(98)00025-8. PMID 11048595.
- El-Saied, Hani M.; Morsi, M. K.; Amer, M. M. A. (June 1981). "Composition of cocoa shell fat as related to cocoa butter". Zeitschrift für Ernährungswissenschaft. 20 (2): 145–151. doi:10.1007/BF02021260. PMID 7269661. S2CID 30329861.
- "USDA nutrient database". Nal.usda.gov. 5 October 2016. Archived from the original on 3 March 2015. Retrieved 3 November 2016.
- Frank, Jill (24 October 2014). "Cocoa Butter Alternatives in Chocolate". Prospector.
- Thomas, Alfred (2002). "Fats and Fatty Oils". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_173. ISBN 978-3-527-30673-2.
- "Cocoa butter prices, bean futures soar". www.foodbusinessnews.net. Retrieved 27 February 2019.
- Moriarty, Andrew. "Cocoa Price: The full story behind the cocoa bean price increase". www.mintecglobal.com. Retrieved 10 March 2022.
- Van Pee, Walter M.; Boni, Luc E.; Foma, Mazibo N.; Hendrikx, Achiel (1981). "Fatty acid composition and characteristics of the kernel fat of different mango (Mangifera indica) varieties". Journal of the Science of Food and Agriculture. 32 (5): 485–488. doi:10.1002/jsfa.2740320510.
- Sonwai, Sopark; Kaphueakngam, Phimnipha; Flood, Adrian (2012). "Blending of mango kernel fat and palm oil mid-fraction to obtain cocoa butter equivalent". Journal of Food Science and Technology. 51 (10): 2357–69. doi:10.1007/s13197-012-0808-7. PMC 4190219. PMID 25328175.
- Chew, Norma (24 November 2011). "What Are The Benefits of Cocoa Butter?". LiveStrong. Retrieved 20 November 2012.
- Skrzypiec, Marcin (12 January 2016). "Can Cocoa Powder Go Bad?". Can It Go Bad?. Retrieved 10 March 2022.
- Wille, R. L.; Lutton, E. S. (1966). "Polymorphism of cocoa butter". Journal of the American Oil Chemists' Society. 43 (8): 491–6. doi:10.1007/BF02641273. PMID 5945032. S2CID 45024885.