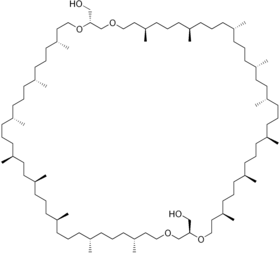
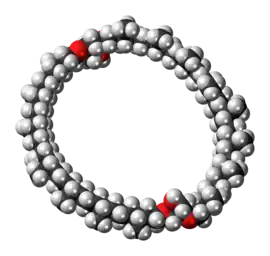
Caldarchaeol
Caldarchaeol is a membrane-spanning lipid of the glycerol dialkyl glycerol tetraether class. It is found in hyperthermophilic archaea. Membranes made up of caldarchaeol are more stable since the hydrophobic chains are linked together, allowing the microorganisms to withstand high temperatures. It is also known as dibiphytanyldiglycerol tetraether. Two glycerol units are linked together by two strains which consist of two phytanes linked together to form a linear chain of 32 carbon atoms (40 carbons including methyl sidechains).
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
[(2R,7R,11R,15S,19S,22S,26S,30R,34R,38R,43R,47R,51S,55S,58S,62S,66R,70R)-7,11,15,19,22,26,30,34,43,47,51,55,58,62,66,70-Hexadecamethyl-1,4,37,40-tetraoxacyclodoheptacontane-2,38-diyl]dimethanol | |
| Other names
Dibiphytanyldiglycerol tetraether | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C86H172O6 | |
| Molar mass | 1302.28 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The configuration of the macrocyclic tetraether has been determined by total synthesis of the C40-diol and comparison with a sample of obtained by degradation of natural tetraether.[1] A synthesis of tetraether has also been carried out.[2]
Notes
- C. H. Heathcock; B. L. Finkelstein; E. T. Jarvi; P. A. Radel; C. R. Hadley (1988). "Acyclic stereoselection. Part 42. 1,4- and 1,5-Stereoselection by sequential aldol addition to a .alpha.,.beta.-unsaturated aldehydes followed by Claisen rearrangement. Application to total synthesis of the vitamin E side chain and the archaebacterial C40 diol". J. Org. Chem. 53 (9): 1922–1942. doi:10.1021/jo00244a017.
- T. Eguchi; K. Ibaragi; K. Kakinuma (1998). "Total Synthesis of Archaeal 72-Membered Macrocyclic Tetraether Lipids". J. Org. Chem. 63 (8): 2689–2698. doi:10.1021/jo972328p. PMID 11672138.
Additional references
- Focus of research, University of Occupational and Environmental Health, Kitakyushu, Japan
- Langworthy TA, Biochim Biophys Acta 1977, 487, 37
- Monolayer properties of archaeol and caldarchaeol polar lipids of a methanogenic archaebacterium, Methanospirillum hungatei, at the air/water interface. Tomoaia-Cotisel M, Chifu E, Zsako J, Mocanu A, Quinn PJ, Kates M. Chem Phys Lipids. 1992 Nov;63(1-2):131-8
- Ether polar lipids of methanogenic bacteria: structures, comparative aspects, and biosyntheses. Koga Y, Nishihara M, Morii H, Akagawa-Matsushita M. Microbiol Rev. 1993 Mar;57(1):164-82.