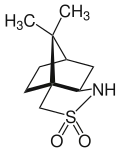
Camphorsultam
Camphorsultam, also known as bornanesultam, is a crystalline solid primarily used as a chiral auxiliary in the synthesis of other chemicals with a specific desired stereoselectivity. Camphorsultam is commercially available in both enantiomers of its exo forms: (1R)-(+)-2,10-camphorsultam and (1S)-(−)-2,10-camphorsultam.
 | |
| Names | |
|---|---|
| IUPAC name
2,10-Camphorsultam | |
| Systematic IUPAC name
(1S,5R,7R)-10,10-Dimethyl-3-thia-4-azatricyclo[5.2.1.01,5]decane 3,3-dioxide | |
| Other names
Camphorsultam, Bornanesultam, Oppolzer's Sultam, (–)-10,2-Camphorsultam, (1S)-(–)-2,10-Camphorsultam, (3aS,6R,7aR)-8,8-Dimethylhexahydro-3a,6-methano-2,1-benzisothiazole 2,2-dioxide, (−)-exo-10,2-Bornanesultam | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C10H17NO2S | |
| Molar mass | 215.31 g·mol−1 |
| Appearance | White, crystalline solid |
| Density | 1.287 g/cm3 |
| Melting point | 181 to 183 °C (358 to 361 °F; 454 to 456 K) |
| Boiling point | 325 °C (617 °F; 598 K) |
Refractive index (nD) |
1.567 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritating to respiratory system and respiratory tract |
| Flash point | 105.3 °C (221.5 °F; 378.4 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
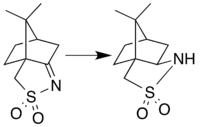
Camphorsultam is synthesized by reduction of camphorsulfonylimine. This reaction was originally performed as a catalytic hydrogenation using Raney Nickel,[2] but the modern preparation instead uses lithium aluminium hydride for the reduction.[3] These reductive methods are stereoselective: although both the endo and exo diastereomeric forms are theoretically possible, only the exo isomer is actually produced due to steric effects of one of the methyl groups.[2] Camphorsultam is often referred to as Oppolzer's sultam in reference to Wolfgang Oppolzer and colleagues, who developed the lithium aluminium hydride approach to this compound and pioneered its use in asymmetric synthesis.[4][5]
Uses
Due to its ability to form derivatives through its nitrogen atom and the structural rigidity of its chirality, camphorsultam is often used in reactions as a chiral auxiliary in order to allow a reaction to proceed with very specific stereoselectivity. During the synthesis of Manzacidin B, camphorsultam is used in order to obtain the desired stereoselective product.[6] During a Michael reaction,[7] a Claisen rearrangement,[8] or a cycloaddition reaction[9] camphorsultam is able to confer a great deal of stereoselectivity. This allows for more control over the reactions and the creation of very specific desired products. Stereoselectivity can be further increased if substrates are equipped with two chiral auxiliaries, acting in a cooperative fashion.[10]
Camphorsultam also has applications in determining a compound's absolute stereochemistry. For that reason, it is sometimes referred to as a "chiral probe".
References
- Chambers, Michael. "ChemIDplus - 0108448777 - DPJYJNYYDJOJNO-UHFFFAOYSA-N - (2S)-Bornane-10,2-sultam - Similar structures search, synonyms, formulas, resource links, and other chemical information". chem.nlm.nih.gov.
- Shriner, R. L.; Shotton, J. A.; Sutherland, H. (1938). "Anomalous Mutarotation of Salts of Reychler's Acid. VI. Synthesis and Structure of the Sultam of 2-(N-Methylamino)-d-camphane-10-sulfonic Acid". J. Am. Chem. Soc. 60 (11): 2794. doi:10.1021/ja01278a072.
- Weismiller, Michael C.; Towson, James C.; Davis, Franklin A. (1990). "(−)-D-2,10-Camphorsultam". Organic Syntheses. 69: 154.; Collective Volume, vol. 8, p. 110
- Oppolzer, Wolfgang."Camphor as a natural source of chirality in asymmetric synthesis".Pure Appl. Chem., Vol. 62, No. 7, pp. 1241-1250, (1990).
- "Archived copy" (PDF). Archived from the original (PDF) on 2010-06-26. Retrieved 2013-07-29.
{{cite web}}: CS1 maint: archived copy as title (link) - Shinada, Tetsuro; Oe, Kentaro; Ohfune, Yasufumi (2012). "Efficient Total Synthesis of Manzacidin B". Tetrahedron Letters. 53 (26): 3250–3253. doi:10.1016/j.tetlet.2012.04.042.
- Tsai, Wen Jiuan; Lin, Yi-Tsong; Uang, Biing-Jiun (1994). "Asymmetric Michael Addition of Thiols to (1R,2R,4R)-(–)-2,10-N-Enoylcamphorsultam". Tetrahedron. 5 (7): 1195–1198. doi:10.1016/0957-4166(94)80155-X.
- Takao, Ken-ichi; Sakamoto, Shu; Touati, Marianne Ayaka; Kusakawa, Yusuke; Tadano, Kin-ichi (2012). "Asmmetric Construction of All-Carbon Quaternary Stereocenters by Chiral Auxiliary Mediated Claisen Rearrangement and Total Synthesis of (+)-Bakuchiol". Molecules. 17 (11): 13330–13344. doi:10.3390/molecules171113330. PMC 6268616. PMID 23138536.
- Romanski, Jan; Nowak, Piotr; Chapuis, Christian; Jurczak, Janusz (2011). "Total synthesis of (5S)-dihydroyashabushiketol". Tetrahedron: Asymmetry. 22 (7): 787–790. doi:10.1016/j.tetasy.2011.04.014.
- Romanski, Jan; Nowak, Piotr; Maksymiuk, Anna; Chapuis, Christian; Jurczak, Janusz (2013). "Diastereoselective 1,3-dipolar cycloadditions of both electronically modified phenyl-nitrile oxides and stilbenes". RSC Advances. 3 (45): 23105–23118. Bibcode:2013RSCAd...323105R. doi:10.1039/C3RA41718B.