Polyacrylic acid
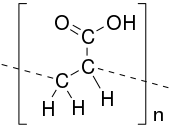
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2-CHCO2H)n. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deprotonated derivatives thereof are known and of commercial value. In a water solution at neutral pH, PAA is an anionic polymer, i.e., many of the side chains of PAA lose their protons and acquire a negative charge. Partially or wholly deprotonated PAAs are polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume. These properties – acid-base and water-attracting – are the bases of many applications.
 | |
| Names | |
|---|---|
| IUPAC name
Poly(acrylic acid), poly(1-carboxyethylene) | |
| Other names
PAA, PAAc, Acrysol, Acumer, Alcosperse, Aquatreat, Carbomer, Sokalan | |
| Identifiers | |
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.115.375 |
| EC Number |
|
| KEGG | |
| UNII |
|
CompTox Dashboard (EPA) |
|
| Properties | |
| (C3H4O2)n | |
| Molar mass | variable |
| log P | 0.25700[1] |
| Hazards[2] | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
PAA is produced by free radical polymerization. Initiators include potassium persulfate and AIBN.
Polyacrylic acid is a polyolefin. It can be viewed as polyethylene with carboxylic acid (CO2H) substituents on alternating carbons. Owing to these groups, alternating carbon atoms in the backbone are stereogenic (colloquially: chiral). For this reason, acrylic acid exists in atactic, syndiotactic, and isotactic forms, although this aspect is rarely discussed. The polymerization is initiated with radicals and is assumed to be stereorandom. Crosslinking can be introduced in many ways.
Production
About 1,600,000,000 kg were produced in 2008.
Structure and derivatives
Polyacrylic acid is a weak anionic polyelectrolyte, whose degree of ionisation is dependent on solution pH. In its non-ionised form at low pHs, PAA may associate with various non-ionic polymers (such as polyethylene oxide, poly-N-vinyl pyrrolidone, polyacrylamide, and some cellulose ethers) and form hydrogen-bonded interpolymer complexes.[3] In aqueous solutions PAA can also form polycomplexes with oppositely charged polymers such as chitosan, surfactants, and drug molecules (for example, streptomycin).[4]
Physical properties
Dry PAAs are sold as white, fluffy powders. In the dry powder form, the positively charged sodium ions are bound to the polyacrylate, however in aqueous solutions the sodium ions can dissociate. The presence of many metal cations allows the polymer to absorb a high amount of water.
Applications
Absorbent
PAA is widely used in dispersants and since the molecular weight has a significant impact on the rheological properties and dispersion capacity, and hence applications. The dominant application for PAA is as a superabsorbent. About 25% of PAA is used for detergents and dispersants.
Polyacrylic acid and its derivatives (particularly sodium polyacrylate) are used in disposable diapers. Acrylic acid is also the main component of Superadsorbent Polymers (SAPs), cross-linked polyacrylates that can absorb and retain more than 100 times of their own weight in liquid. The US Food and Drug Administration authorised the use of SAPs in packaging with indirect food contact.[5][6]
Cleaning
Detergents often contain copolymers of acrylic acid that assist in sequestering dirt. Cross-linked polyacrylic acid has also been used in the processing of household products, including floor cleaners. PAA may inactivate the antiseptic chlorhexidine gluconate.[7]
Biocompatible materials
The neutralized polyacrylic acid gels are suitable biocompatible matrices for medical applications such as gels for skin care products. PAA films can be deposited on orthopaedic implants to protect them from corrosion. Crosslinked hydrogels of PAA and gelatin have also been used as medical glue.
Paints and cosmetics
Other applications involve paints and cosmetics. They stabilize suspended solid in liquids, prevent emulsions from separating, and control the consistency in flow of cosmetics. Carbomer codes (910, 934, 940, 941, and 934P) are an indication of molecular weight and the specific components of the polymer. For many applications PAAs are used in form of alkali metal or ammonium salts, e.g. sodium polyacrylate.
Emerging applications
Hydrogels derived from PAA have attracted much study for use as bandages and aids for wound healing.[8]
Drilling fluid and metal quenching
A few reports were made on PAA use as deflocculant (so called alkaline polyacrylates) for oil drilling industry.[9][10]
It was also reported to be used for metal quenching in metalworking (see Sodium polyacrylate).[11]
References
- "Polyacrylic acid_msds". Archived from the original on 2022-02-21. Retrieved 2018-04-23.
- "C&L Inventory". echa.europa.eu. Archived from the original on 2021-04-05. Retrieved 2021-12-05.
- Khutoryanskiy, Vitaliy V.; Staikos, Georgios (9 March 2009). Hydrogen-bonded Interpolymer Complexes: Formation, Structure And Applications. World Scientific. ISBN 978-981-4475-04-4. OCLC 1200871469. Archived from the original on 2022-02-21. Retrieved 2022-02-21.
- Nurkeeva, Zauresh S; Khutoryanskiy, Vitaliy V; Mun, Grigoriy A; Sherbakova, Marina V; Ivaschenko, Anatoliy T; Aitkhozhina, Nazira A (March 2004). "Polycomplexes of poly(acrylic acid) with streptomycin sulfate and their antibacterial activity". European Journal of Pharmaceutics and Biopharmaceutics. 57 (2): 245–9. doi:10.1016/S0939-6411(03)00149-8. PMID 15018981. Archived from the original on 21 February 2022 – via PubMed, Elsevier.
- Orwoll, Robert A.; Yong, Chong S. (1999). "Poly(acrylic acid)". In Mark, James E. (ed.). Polymer Data Handbook. Oxford University Press, Inc. pp. 252–253. ISBN 978-0195107890. OCLC 39962426. Archived from the original on 2022-02-21. Retrieved 2022-02-21.
- "Acrylates". The Macrogalleria. Polymer Science Learning Center. 2005. Archived from the original on 21 February 2022. Retrieved 25 June 2015.
- Kaiser, Nancy; Klein, Dan; Karanja, Peter; Greten, Zachariah; Newman, Jerry (2009). "Inactivation of chlorhexidine gluconate on skin by incompatible alcohol hand sanitizing gels". American Journal of Infection Control. 37 (7): 569–73. doi:10.1016/j.ajic.2008.12.008. PMID 19398245. Archived from the original on 19 December 2021 – via PubMed, Elsevier.
- Mogoşanu, George Dan; Grumezescu, Alexandru Mihai (25 March 2014). "Natural and synthetic polymers for wounds and burns dressing". International Journal of Pharmaceutics. 463 (2): 127–136. doi:10.1016/j.ijpharm.2013.12.015. PMID 24368109. Archived from the original on 1 February 2022.
- "Deflocculants: A Detailed Overview". Archived from the original on February 26, 2021.
- Petrov, N.A.; Maikobi, A.A. (December 2017). "INVESTIGATION OF UNIFLOX REAGENT FOR DRILLING SIBERIAN SOLVENT SOLUTIONS". Oil and Gas Business (6): 6–19. doi:10.17122/ogbus-2017-6-6-19.
- Griffiths, W. D. (1989). The quenching characteristics of sodium polyacrylate solutions (doctoral thesis). Sheffield: Sheffield Hallam University.


