Carbonic anhydrase 4
Carbonic anhydrase 4 is an enzyme that in humans is encoded by the CA4 gene.[5][6]
Function
Carbonic anhydrases (CAs) are a large family of zinc metalloenzymes that catalyze the reversible hydration of carbon dioxide. They participate in a variety of biological processes, including respiration, calcification, acid-base balance, bone resorption, and the formation of aqueous humor, cerebrospinal fluid, saliva, and gastric acid. They show extensive diversity in tissue distribution and in their subcellular localization. CA IV is a glycosylphosphatidyl-inositol-anchored membrane isozyme expressed on the luminal surfaces of pulmonary (and certain other) capillaries and of proximal renal tubules. Its exact function is not known, however, it may have a role in inherited renal abnormalities of bicarbonate transport.[6]
CA IV has been identified in pulmonary epithelium of many mammalian species and may be uniquely adaptive for gas exchange necessary for the high metabolic requirements of mammals. A majority of the CO2 produced by metabolism is transported as bicarbonate (HCO−
3). At the tissue capillary, CO2 diffuses from tissue to plasma. Other forms of carbonic anhydrase enzyme are not present in the plasma, restricting the equilibrium reaction of CO2+H2O = H
2CO
3 = H+ HCO−
3. CO2 in the plasma diffuses into the Red Blood Cell. CA is present within the Red Blood Cell, facilitating the conversion of CO2 to HCO−
3. HCO−
3 so produced is transferred by the HCO−
3/Cl- "shuttle" from the interior of the Red Blood Cell to the plasma. HCO−
3 does not diffuse across cell membranes and, in the absence of CA, stays as HCO−
3 and concentrates in plasma. Up to 80% of metabolically produced CO2 is transported in plasma in the form of HCO−
3. Blood moves from the tissue capillary to the pulmonary capillary where CO2 is exchanged at the lung. In the pulmonary capillary, bicarbonate can not simply diffuse either into the Red Blood Cell or the alveoli. It is traditionally thought that HCO−
3 is returned to the interior of the Red Blood Cell by a reversal of the HCO−
3/Cl- shuttle, where, in the presence of CA, it is returned to a CO2 form to diffuse from the interior of the Red Blood Cell, to the plasma and then into the alveoli. Membrane bound CA (CA IV) on the luminal side of the pulmonary membrane would have direct contact with plasma HCO−
3 and would enzymatically convert HCO−
3 to CO2 in the area immediately proximal to the exchange membrane, greatly increasing the concentration gradient for exchange. In this way, plasma HCO−
3 can be converted to CO2 within the plasma compartment and exchanged with the alveoli without the requirement of returning the HCO−
3 to the interior of the Red Blood Cell.
References
- GRCh38: Ensembl release 89: ENSG00000167434 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000000805 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Okuyama T, Batanian JR, Sly WS (Aug 1993). "Genomic organization and localization of gene for human carbonic anhydrase IV to chromosome 17q". Genomics. 16 (3): 678–84. doi:10.1006/geno.1993.1247. PMID 8325641.
- "Entrez Gene: CA4 carbonic anhydrase IV".
- Sterling D, Alvarez BV, Casey JR (Jul 2002). "The extracellular component of a transport metabolon. Extracellular loop 4 of the human AE1 Cl-/HCO3- exchanger binds carbonic anhydrase IV". J. Biol. Chem. 277 (28): 25239–46. doi:10.1074/jbc.M202562200. PMID 11994299.
Send to
Further reading
- Okuyama T, Sato S, Zhu XL, Waheed A, Sly WS (1992). "Human carbonic anhydrase IV: cDNA cloning, sequence comparison, and expression in COS cell membranes". Proc. Natl. Acad. Sci. U.S.A. 89 (4): 1315–9. Bibcode:1992PNAS...89.1315O. doi:10.1073/pnas.89.4.1315. PMC 48440. PMID 1311094.
- Hageman GS, Zhu XL, Waheed A, Sly WS (1991). "Localization of carbonic anhydrase IV in a specific capillary bed of the human eye". Proc. Natl. Acad. Sci. U.S.A. 88 (7): 2716–20. Bibcode:1991PNAS...88.2716H. doi:10.1073/pnas.88.7.2716. PMC 51309. PMID 1901414.
- Zhu XL, Sly WS (1990). "Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney". J. Biol. Chem. 265 (15): 8795–801. doi:10.1016/S0021-9258(19)38958-6. PMID 2111324.
- Carter ND, Fryer A, Grant AG, Hume R, Strange RG, Wistrand PJ (1990). "Membrane specific carbonic anhydrase (CAIV) expression in human tissues". Biochim. Biophys. Acta. 1026 (1): 113–6. doi:10.1016/0005-2736(90)90340-T. PMID 2116168.
- Whitney PL, Briggle TV (1982). "Membrane-associated carbonic anhydrase purified from bovine lung". J. Biol. Chem. 257 (20): 12056–9. doi:10.1016/S0021-9258(18)33676-7. PMID 6811592.
- Bardien S, Ebenezer N, Greenberg J, Inglehearn CF, Bartmann L, Goliath R, Beighton P, Ramesar R, Bhattacharya SS (1995). "An eighth locus for autosomal dominant retinitis pigmentosa is linked to chromosome 17q". Hum. Mol. Genet. 4 (8): 1459–62. doi:10.1093/hmg/4.8.1459. PMID 7581389.
- Okuyama T, Waheed A, Kusumoto W, Zhu XL, Sly WS (1995). "Carbonic anhydrase IV: role of removal of C-terminal domain in glycosylphosphatidylinositol anchoring and realization of enzyme activity". Arch. Biochem. Biophys. 320 (2): 315–22. doi:10.1016/0003-9861(95)90015-2. PMID 7625839.
- Mahieu I, Benjamin A, Stephens R, Walters D, Carter N (1995). "Characterization of membrane bound carbonic anhydrase IV (CA IV) located on the external surface of lung pulmonary endothelial cells". Biochem. Soc. Trans. 23 (2): 320S. doi:10.1042/bst023320s. PMID 7672351.
- Sender S, Gros G, Waheed A, Hageman GS, Sly WS (1994). "Immunohistochemical localization of carbonic anhydrase IV in capillaries of rat and human skeletal muscle". J. Histochem. Cytochem. 42 (9): 1229–36. doi:10.1177/42.9.8064130. PMID 8064130.
- Fleming RE, Parkkila S, Parkkila AK, Rajaniemi H, Waheed A, Sly WS (1996). "Carbonic anhydrase IV expression in rat and human gastrointestinal tract regional, cellular, and subcellular localization". J. Clin. Invest. 96 (6): 2907–13. doi:10.1172/JCI118362. PMC 186002. PMID 8675662.
- Waheed A, Okuyama T, Heyduk T, Sly WS (1996). "Carbonic anhydrase IV: purification of a secretory form of the recombinant human enzyme and identification of the positions and importance of its disulfide bonds". Arch. Biochem. Biophys. 333 (2): 432–8. doi:10.1006/abbi.1996.0412. PMID 8809084.
- Parkkila S, Parkkila AK, Juvonen T, Waheed A, Sly WS, Saarnio J, Kaunisto K, Kellokumpu S, Rajaniemi H (1996). "Membrane-bound carbonic anhydrase IV is expressed in the luminal plasma membrane of the human gallbladder epithelium". Hepatology. 24 (5): 1104–8. doi:10.1002/hep.510240521. PMID 8903383. S2CID 11461543.
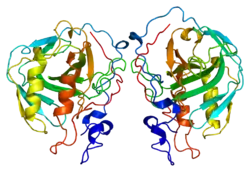
- Stams T, Nair SK, Okuyama T, Waheed A, Sly WS, Christianson DW (1997). "Crystal structure of the secretory form of membrane-associated human carbonic anhydrase IV at 2.8-A resolution". Proc. Natl. Acad. Sci. U.S.A. 93 (24): 13589–94. doi:10.1073/pnas.93.24.13589. PMC 19359. PMID 8942978.
- Bardien S, Ramesar R, Bhattacharya S, Greenberg J (1997). "Retinitis pigmentosa locus on 17q (RP17): fine localization to 17q22 and exclusion of the PDEG and TIMP2 genes". Hum. Genet. 101 (1): 13–7. doi:10.1007/s004390050577. PMID 9385361. S2CID 26680917.
- Sender S, Decker B, Fenske CD, Sly WS, Carter ND, Gros G (1998). "Localization of carbonic anhydrase IV in rat and human heart muscle". J. Histochem. Cytochem. 46 (7): 855–61. doi:10.1177/002215549804600709. PMID 9632745.
- Wistrand PJ, Carter ND, Conroy CW, Mahieu I (1999). "Carbonic anhydrase IV activity is localized on the exterior surface of human erythrocytes". Acta Physiol. Scand. 165 (2): 211–8. doi:10.1046/j.1365-201x.1999.00478.x. PMID 10090333.
- Fujikawa-Adachi K, Nishimori I, Sakamoto S, Morita M, Onishi S, Yonezawa S, Hollingsworth MA (1999). "Identification of carbonic anhydrase IV and VI mRNA expression in human pancreas and salivary glands". Pancreas. 18 (4): 329–35. doi:10.1097/00006676-199905000-00001. PMID 10231836.
- Sterling D, Alvarez BV, Casey JR (2002). "The extracellular component of a transport metabolon. Extracellular loop 4 of the human AE1 Cl-/HCO3- exchanger binds carbonic anhydrase IV". J. Biol. Chem. 277 (28): 25239–46. doi:10.1074/jbc.M202562200. PMID 11994299.
External links
- GeneReviews/NCBI/NIH/UW entry on Retinitis Pigmentosa Overview
- Overview of all the structural information available in the PDB for UniProt: P22748 (Carbonic anhydrase 4) at the PDBe-KB.