Centrin
Centrins, also known as caltractins, are a family of calcium-binding phosphoproteins found in the centrosome of eukaryotes.[2][3] Centrins are small calcium binding proteins that are ubiquitous centrosome components. There are about 350 “signature” proteins that are unique to eukaryotic cells but have no significant homology to proteins in archaea and bacteria.[4] They are a type of protein that is essential and present in almost all eukaryotic cells and are found in the centrioles and pericentriolar lattice.[5] Human centrin genes are CETN1, CETN2 and CETN3.[2][3]
| Caltractin | |||||||
|---|---|---|---|---|---|---|---|
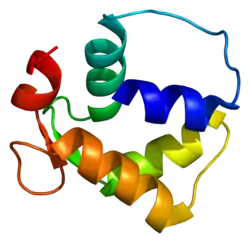
 Crystal structure of the SdCen/skMLCK complex.[1] | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | caltractin | ||||||
| RefSeq (mRNA) | X69220 | ||||||
| UniProt | Q06827 | ||||||
| |||||||
Humans and mice have three centrin genes: Cetn-1, which is typically only expressed in male germ cells, and Cetn-2 and Cetn-3, which are typically only expressed in somatic cells. Centrin-2 is a recombinant GFP-centrin-2 and centriole protein that localizes to centrioles throughout the cell cycle, while centrin-3 seems to stick to the pericentriolar material that surrounds the centrioles.[5]
History
Centrin was first isolated and characterized from the flagellar roots of the green alga Tetraselmis striata in 1984.[6] Jeffrey Salisbury, who discovered centrin in the green algae, and his colleagues used RNA interference (RNAi) to reduce the levels of centrin-2 in human tissue culture cells. The RNAi of centrin-2 from HeLa cells had led to progressive losses in the centrioles and was consistent with full blocks in the centriole replication. He had proved that centrin was involved in centriole duplication in animal cells like seen in his previous work with algae. This implies that centrin requirement was absolute for plants and animals within the centriole.[7]
Function
Centrins are required for duplication of centrioles.[3] They may also play a role in severing of microtubules by causing calcium-mediated contraction.[8] It was found that centrin was essential within the calcium channel metabolism and it has a high affinity for calcium and a way lower affinity for phosphorus and other cell mineral constituents.[5] Centrins show calcium-sensitive contractile behavior and was identified before as a calcium sensing regulator of the centriole structure.[9] It is one of the first proteins to localize at sites of newly forming centrioles in semiconservative and novo assembly pathways. In algae, ciliates, and lower land plants failure of centrioles to duplicate is shown when a mutation, deletion, or knockdown of centrin happens by RNAi because centrin is a key factor for the structural integrity of centrioles.[10]
Studies of experimental ablation of centrin synthesis in alga Chlamydomonas cryptogamous water fern Marsilea indicate a key role of centrin having to do with centriole biogenesis.
Centrins facilitated the duplication of centrioles and the severing of microtubules by calcium mediated contraction. The centrin found was highly concentrated outside of the centrosome and a lot of it was found to be non-centrosomal, which assembled during meiosis two.[11] The extra-centrosomal materials function is not yet fully understood by researchers yet but using cross linking found centrin does have an affinity for actin and the terminal portion of the HC. Immunoprecipitation assays are needed in order to confirm this.[5]
Structure
Centrin belongs to the EF-hand superfamily of calcium-binding proteins and has four calcium-binding EF-hands.[12] It has a molecular weight of 20 kDa.[13]
Centrins contain four helix-loop-helix features specifically made binding with calcium in the transitional region of the axoneme. The axoneme is the bridge between the nucleus and the basal body where the proximal and distal fibers are connecting two basal bodies. Centrin is also present in the set of fiberd that connect the microtubule blades.[5]
Studies of higher eukaryotic cells such as human cells proved that centrins are the universal centrosome protein that occurs in fibers linking centrioles to one another and the distal most core structure called the "transition zone".[10]
Centrin present distal molecular strings which contract at different speeds and connect two cilia and the nuclear-basal body connectors. These respond in a continuous analog fashion to the internal calcium concentration mechanism which will set up a great spot at the base for cells cilia to attach to the cells body.[14]
See also
References
- "RCSB Protein Data Bank - Structure Summary for 3KF9 - Crystal structure of the SdCen/skMLCK complex".
- Baron, A. T.; Greenwood, T. M.; Bazinet, C. W.; Salisbury, J. L. (1992). "Centrin is a component of the pericentriolar lattice". Biology of the Cell. 76 (3): 383–388. doi:10.1016/0248-4900(92)90442-4. PMID 1305481. S2CID 85374106.
- Salisbury JL, Suino KM, Busby R, Springett M (2002). "Centrin-2 is required for centriole duplication in mammalian cells". Curr. Biol. 12 (15): 1287–92. doi:10.1016/S0960-9822(02)01019-9. PMID 12176356. S2CID 1415623.
- Salisbury, Jeffery L (2002). "Centrin-2 Is required for Centriole Duplication in Mammalian Cells". Current Biology. 12 (15): 1287–1292. doi:10.1016/s0960-9822(02)01019-9. PMID 12176356. S2CID 1415623.
- "Centrin Protein | CETN1 Peptide | CETN2 Antigen | ProSpec". www.prospecbio.com. Retrieved 2023-05-02.
- Salisbury JL, Baron A, Surek B, Melkonian M (1984). "Striated flagellar roots: isolation and partial characterization of a calcium-modulated contractile organelle". J. Cell Biol. 99 (3): 962–70. doi:10.1083/jcb.99.3.962. PMC 2113404. PMID 6381510.
- Laporte, Marine H; Bouhlel, Imène B; Bertiaux, Eloïse; Morrison, Ciaran G; Giroud, Alexia; Borgers, Susanne; Azimzadeh, Juliette; Bornens, Michel; Guichard, Paul; Paoletti, Anne; Hamel, Virginie (2022-11-02). "Human SFI1 and Centrin form a complex critical for centriole architecture and ciliogenesis". The EMBO Journal. 41 (21): e112107. doi:10.15252/embj.2022112107. ISSN 0261-4189. PMC 9627676. PMID 36125182.
- Wolfrum, U. (1995). "Centrin in the photoreceptor cells of mammalian retinae". Cell Motility and the Cytoskeleton. 32 (1): 55–64. doi:10.1002/cm.970320107. PMID 8674134.
- academic.oup.com https://academic.oup.com/femsle/article/233/1/91/460135. Retrieved 2023-05-02.
{{cite web}}: Missing or empty|title=(help) - Salisbury, Jeffrey L. (November 2007). "A mechanistic view on the evolutionary origin for centrin-based control of centriole duplication". Journal of Cellular Physiology. 213 (2): 420–428. doi:10.1002/jcp.21226. PMID 17694534. S2CID 43032704.
- Tillery, Marisa M. L.; Blake-Hedges, Caitlyn; Zheng, Yiming; Buchwalter, Rebecca A.; Megraw, Timothy L. (2018-08-28). "Centrosomal and Non-Centrosomal Microtubule-Organizing Centers (MTOCs) in Drosophila melanogaster". Cells. 7 (9): 121. doi:10.3390/cells7090121. ISSN 2073-4409. PMC 6162459. PMID 30154378.
- Salisbury JL (1995). "Centrin, centrosomes, and mitotic spindle poles". Curr. Opin. Cell Biol. 7 (1): 39–45. doi:10.1016/0955-0674(95)80043-3. PMID 7755988.
- Levy YY, Lai EY, Remillard SP, Heintzelman MB, Fulton C (1996). "Centrin is a conserved protein that forms diverse associations with centrioles and MTOCs in Naegleria and other organisms". Cell Motil. Cytoskeleton. 33 (4): 298–323. doi:10.1002/(SICI)1097-0169(1996)33:4<298::AID-CM6>3.0.CO;2-5. PMID 8801035.
- "Centrin - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2023-05-02.