Pleomorphic adenoma
Pleomorphic adenoma (or benign mixed tumor) is a common benign salivary gland neoplasm characterised by neoplastic proliferation of epithelial (ductal) cells along with myoepithelial components, having a malignant potentiality. It is the most common type of salivary gland tumor and the most common tumor of the parotid gland. It derives its name from the architectural Pleomorphism (variable appearance) seen by light microscopy. It is also known as "Mixed tumor, salivary gland type", which refers to its dual origin from epithelial and myoepithelial elements as opposed to its pleomorphic appearance.
| Pleomorphic adenoma | |
|---|---|
| Other names | Benign mixed tumor[1] |
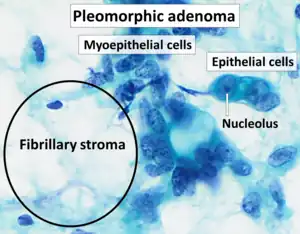 | |
| Cytopathology of pleomorphic adenoma (Pap stain). It can usually be diagnosed by its typical fibrillary stroma (mesenchyme). Stromal cell nuclei are small. Myoepithelial cells are usually the predominant cell type, and their nuclei can have various shapes but are usually more elongated than in epithelial cells. Epithelial cell nuclei may have prominent nucleoli.[2] | |
| Specialty | Oncology |
Clinical presentation
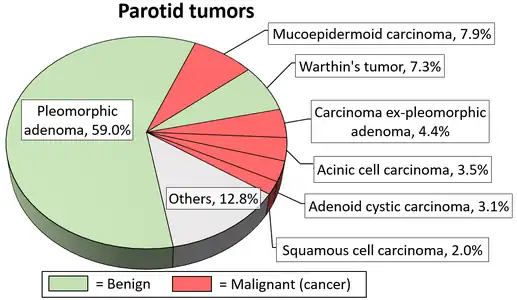
The tumor is usually solitary and presents as a slow growing, painless, firm single nodular mass. Isolated nodules are generally outgrowths of the main nodule rather than a multinodular presentation. It is usually mobile unless found in the palate and can cause atrophy of the mandibular ramus when located in the parotid gland. When found in the parotid tail, it may present as an eversion of the ear lobe. Though it is classified as a benign tumor, pleomorphic adenomas have the capacity to grow to large proportions and may undergo malignant transformation, to form carcinoma ex-pleomorphic adenoma, a risk that increases with time (9.5% chance to convert into malignancy in 15 years). Although it is "benign", the tumor is aneuploid, it can recur after resection, it invades normal adjacent tissue, and distant metastases have been reported after long (+10 years) time intervals. This tumour most often presents in the lower pole of the superficial lobe of the gland, about 10% of the tumours arise in the deeper portions of the gland. It occurs more frequently in females than in males, the ratio approximating 6:4. The majority of the lesion are found in patients in the fourth to sixth decades with an average age of occurrences of about 43 years, but these are relatively common in young adults and have been known to occur in children.
Histology

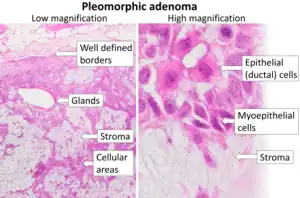
Morphological diversity is the most characteristic feature of this neoplasm. Histologically, it is highly variable in appearance, even within individual tumors. Classically it is biphasic and is characterized by an admixture of polygonal epithelial and spindle-shaped myoepithelial elements in a variable background stroma that may be mucoid, myxoid, cartilaginous or hyaline. Epithelial elements may be arranged in duct-like structures, sheets, clumps and/or interlacing strands and consist of polygonal, spindle or stellate-shaped cells (hence pleiomorphism). Areas of squamous metaplasia and epithelial pearls may be present. The tumor is not enveloped, but it is surrounded by a fibrous pseudocapsule of varying thickness. The tumor extends through normal glandular parenchyma in the form of finger-like pseudopodia, but this is not a sign of malignant transformation.
The tumor often displays characteristic chromosomal translocations between chromosomes #3 and #8. This causes the PLAG gene to be juxtaposed to the gene for beta catenin. This activates the catenin pathway and leads to inappropriate cell division.
Diagnosis
The diagnosis of salivary gland tumors utilize both tissue sampling and radiographic studies. Tissue sampling procedures include fine needle aspiration (FNA) and core needle biopsy (bigger needle comparing to FNA). Both of these procedures can be done in an outpatient setting. Diagnostic imaging techniques for salivary gland tumors include ultrasound, computer tomography (CT) and magnetic resonance imaging (MRI).
Fine needle aspiration biopsy (FNA), operated in experienced hands, can determine whether the tumor is malignant in nature with sensitivity around 90%.[3][4] FNA can also distinguish primary salivary tumor from metastatic disease.
Core needle biopsy can also be done in outpatient setting. It is more invasive but is more accurate compared to FNA with diagnostic accuracy greater than 97%.[5] Furthermore, core needle biopsy allows more accurate histological typing of the tumor.
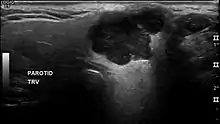
In terms of imaging studies, ultrasound can determine and characterize superficial parotid tumors. Certain types of salivary gland tumors have certain sonographic characteristics on ultrasound.[6] Ultrasound is also frequently used to guide FNA or core needle biopsy.
CT allows direct, bilateral visualization of the salivary gland tumor and provides information about overall dimension and tissue invasion. CT is excellent for demonstrating bony invasion. MRI provides superior soft tissue delineation such as perineural invasion when compared to CT only.[7]
Treatment

Overall, the mainstay of the treatment for salivary gland tumor is surgical resection.[9] Needle biopsy is highly recommended prior to surgery to confirm the diagnosis. More detailed surgical technique and the support for additional adjuvant radiotherapy depends on whether the tumor is malignant or benign.
Surgical treatment of parotid gland tumors is sometimes difficult, partly because of the anatomical relationship of the facial nerve and the parotid lodge, but also through the increased potential for postoperative relapse.[9][10][11] Thus, detection of early stages of a tumor of the parotid gland is extremely important in terms of prognosis after surgery.[12]
There have been several approaches for surgery of parotid pleomorphic adenoma in the course of time. Enucleation of the tumor (i.e. intracapsular dissection), a procedure that was common in the early 20th century, is nowadays obsolete due to very high incidence of recurrence.[9] After the time of enucleations, pleomorphic adenomas of parotid gland were recommended to be routinely treated with superficial or total parotidectomy.[13] These procedures combine complete tumor removal and identification of the main trunk of facial nerve during surgery to avoid any lesions to the nerve. However, extensive surgery may cause significant morbidity, such as Frey´s syndrome (excessive sweat while eating) and salivary fistula.[14][15] Also, aesthetic outcome may be compromised. Therefore, less invasive procedures have been preferred in selected cases during the recent years, and introduction of perioperative neuromonitoring enabled the evolution of several different surgical techniques some twenty years ago.[9][16]
Currently, the choice of surgical approach for parotid pleomorphic adenoma is mainly based on the size, location, and mobility of the tumor. The recommended main techniques include extracapsular dissection, partial superficial parotidectomy, and lateral or total parotidectomy. Nevertheless, the experience of surgeon plays a key role in the results of these distinct procedures.[9] An important point of view is that recurrent pleomorphic adenomas may occur after a very long time from primary surgery, on average over 7–10 years but up to 24 years afterwards.[11][10] Thus, it is of utmost importance to evaluate the ultimate results of these different surgical techniques in the future.
The benign tumors of the submandibular gland is treated by simple excision with preservation of mandibular branch of the facial nerve, the hypoglossal nerve, and the lingual nerve.[17] Other benign tumors of minor salivary glands are treated similarly.
Malignant salivary tumors usually require wide local resection of the primary tumor. However, if complete resection cannot be achieved, adjuvant radiotherapy should be added to improve local control.[18][19] This surgical treatment has many sequelae such as cranial nerve damage, Frey's syndrome, cosmetic problems, etc.
Usually about 44% of the patients have a complete histologic removal of the tumor and this refers to the most significant survival rate.
See also
- Warthin's tumor - monomorphic adenoma
- Carcinoma
- Sialadenitis
References
- Bin Xu, M.D., Ph.D. "Pleomorphic adenoma". Pathology Outlines.
{{cite web}}: CS1 maint: multiple names: authors list (link) Last author update: 30 July 2021. Last staff update: 6 February 2023 - Image by Mikael Häggström, MD. Reference for description: Bin Xu, M.D., Ph.D. "Pleomorphic adenoma". Pathology Outlines.
{{cite web}}: CS1 maint: multiple names: authors list (link) Last author update: 30 July 2021. Last staff update: 6 February 2023 - Cohen EG, Patel SG, Lin O, Boyle JO, Kraus DH, Singh B, et al. (June 2004). "Fine-needle aspiration biopsy of salivary gland lesions in a selected patient population". Archives of Otolaryngology–Head & Neck Surgery. 130 (6): 773–778. doi:10.1001/archotol.130.6.773. PMID 15210562.
- Batsakis JG, Sneige N, el-Naggar AK (February 1992). "Fine-needle aspiration of salivary glands: its utility and tissue effects". The Annals of Otology, Rhinology, and Laryngology. 101 (2 Pt 1): 185–188. doi:10.1177/000348949210100215. PMID 1739267. S2CID 10583105.
- Wan YL, Chan SC, Chen YL, Cheung YC, Lui KW, Wong HF, et al. (October 2004). "Ultrasonography-guided core-needle biopsy of parotid gland masses". AJNR. American Journal of Neuroradiology. 25 (9): 1608–1612. PMC 7976420. PMID 15502149.
- Białek EJ, Jakubowski W, Karpińska G (September 2003). "Role of ultrasonography in diagnosis and differentiation of pleomorphic adenomas: work in progress". Archives of Otolaryngology–Head & Neck Surgery. 129 (9): 929–933. doi:10.1001/archotol.129.9.929. PMID 12975263.
- Koyuncu M, Seşen T, Akan H, Ismailoglu AA, Tanyeri Y, Tekat A, et al. (December 2003). "Comparison of computed tomography and magnetic resonance imaging in the diagnosis of parotid tumors". Otolaryngology–Head and Neck Surgery. 129 (6): 726–732. doi:10.1016/j.otohns.2003.07.009. PMID 14663442. S2CID 24528326.
- Steve C Lee, MD, PhD. "Salivary Gland Neoplasms". Medscape.
{{cite web}}: CS1 maint: multiple names: authors list (link) Updated: Jan 13, 2021
Diagrams by Mikael Häggström, MD - Psychogios G, Bohr C, Constantinidis J, Canis M, Vander Poorten V, Plzak J, et al. (January 2021). "Review of surgical techniques and guide for decision making in the treatment of benign parotid tumors" (PDF). European Archives of Oto-Rhino-Laryngology. 278 (1): 15–29. doi:10.1007/s00405-020-06250-x. PMID 32749609. S2CID 220965351.
- Schapher M, Koch M, Agaimy A, Goncalves M, Mantsopoulos K, Iro H (September 2019). "Parotid pleomorphic adenomas: Factors influencing surgical techniques, morbidity, and long-term outcome relative to the new ESGS classification in a retrospective study". Journal of Cranio-Maxillo-Facial Surgery. 47 (9): 1356–1362. doi:10.1016/j.jcms.2019.06.009. PMID 31331850. S2CID 198169972.
- Silvoniemi A, Pulkkinen J, Grénman R (November 2010). "Parotidectomy in the treatment of pleomorphic adenoma -- analysis of long-term results". Acta Oto-Laryngologica. 130 (11): 1300–1305. doi:10.3109/00016489.2010.488248. PMID 20528201. S2CID 20865807.
- Alexandru Bucur; Octavian Dincă; Tiberiu Niță; Cosmin Totan; Cristian Vlădan (March 2011). "Parotid tumors: our experience". Rev. chir. oro-maxilo-fac. implantol. (in Romanian). 2 (1): 7–9. ISSN 2069-3850. 18. Archived from the original on 2013-01-13. Retrieved 2012-06-06.(webpage has a translation button)
- Patey DH, Thackray AC (March 1958). "The treatment of parotid tumours in the light of a pathological study of parotidectomy material". The British Journal of Surgery. 45 (193): 477–487. doi:10.1002/bjs.18004519314. PMID 13536351. S2CID 24930522.
- Mantsopoulos K, Koch M, Klintworth N, Zenk J, Iro H (January 2015). "Evolution and changing trends in surgery for benign parotid tumors". The Laryngoscope. 125 (1): 122–127. doi:10.1002/lary.24837. PMID 25043324. S2CID 1929744.
- Britt CJ, Stein AP, Gessert T, Pflum Z, Saha S, Hartig GK (February 2017). Eisele DW (ed.). "Factors influencing sialocele or salivary fistula formation postparotidectomy". Head & Neck. 39 (2): 387–391. doi:10.1002/hed.24564. PMID 27550745. S2CID 3758029.
- McGurk M, Thomas BL, Renehan AG (November 2003). "Extracapsular dissection for clinically benign parotid lumps: reduced morbidity without oncological compromise". British Journal of Cancer. 89 (9): 1610–1613. doi:10.1038/sj.bjc.6601281. PMC 2394403. PMID 14583757.
- Leonetti JP, Marzo SJ, Petruzzelli GJ, Herr B (September 2005). "Recurrent pleomorphic adenoma of the parotid gland". Otolaryngology–Head and Neck Surgery. 133 (3): 319–322. doi:10.1016/j.otohns.2005.04.008. PMID 16143173. S2CID 38480631.
- Ganly I, Patel SG, Coleman M, Ghossein R, Carlson D, Shah JP (July 2006). "Malignant minor salivary gland tumors of the larynx". Archives of Otolaryngology–Head & Neck Surgery. 132 (7): 767–770. doi:10.1001/archotol.132.7.767. PMID 16847187.
- Terhaard CH, Lubsen H, Rasch CR, Levendag PC, Kaanders HH, Tjho-Heslinga RE, et al. (January 2005). "The role of radiotherapy in the treatment of malignant salivary gland tumors". International Journal of Radiation Oncology, Biology, Physics. 61 (1): 103–111. doi:10.1016/j.ijrobp.2004.03.018. PMID 15629600.