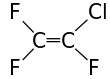
Chlorotrifluoroethylene
Chlorotrifluoroethylene (CTFE) is a chlorofluorocarbon with chemical formula CFCl=CF2. It is commonly used as a refrigerant in cryogenic applications. CTFE has a carbon-carbon double bond and so can be polymerized to form polychlorotrifluoroethylene or copolymerized to produce the plastic ECTFE. PCTFE has the trade name Neoflon PCTFE from Daikin Industries in Japan, and it used to be produced under the trade name Kel-F from 3M Corporation in Minnesota.[2]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1-Chloro-1,2,2-trifluoroethene | |||
| Other names
Chlorotrifluoroethene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.001.093 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1082 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2ClF3 | |||
| Molar mass | 116.47 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | faint etheral odor | ||
| Density | 1.54 g/cm3 at −60°C | ||
| Melting point | −158.2 °C (−252.8 °F; 115.0 K) | ||
| Boiling point | −27.8 °C (−18.0 °F; 245.3 K) | ||
| 4.01 g/100 mL | |||
| Solubility | soluble in benzene, chloroform | ||
| -49.1·10−6 cm3/mol | |||
Refractive index (nD) |
1.38 (0 °C) | ||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Danger | |||
| H220, H280, H301, H331 | |||
| P210, P261, P264, P270, P271, P301+P310, P304+P340, P311, P321, P330, P377, P381, P403, P403+P233, P405, P410+P403, P501 | |||
| NFPA 704 (fire diamond) | |||
| Explosive limits | 24-40.3% | ||
| Related compounds | |||
Related compounds |
Tetrafluoroethylene Bromotrifluoroethylene Trifluoroiodoethylene Dichlorodifluoroethylene Trichlorofluoroethylene Tetrachloroethylene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Production and reactions
Chlorotrifluoroethylene is produced commercially by the dechlorination of 1,1,2-trichloro-1,2,2-trifluoroethane with zinc:[3]
- CFCl2-CF2Cl + Zn → CClF=CF2 + ZnCl2
In 2012, an estimated 1–10 million pounds were produced commercially in the United States.
The thermal dimerization of chlorotrifluoroethylene gives 1,2-dichloro-1,2,3,3,4,4-hexafluorocyclobutane.[4] Dichlorination of the latter gives hexafluorocyclobutene. It undergoes [2+2] cycloaddition to vinyl acetate.[5]
References
- Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 3–126. ISBN 0-8493-0594-2.
- Aetna Plastics Corp. - Products. Services ... Solutions, Aetna Plastics Corp., pp. PCTFE / Kel–F® / Neoflon®, archived from the original on 27 November 2022, retrieved 3 February 2012
- Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2002). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349.
- Buxton, M. W.; Ingram, D. W.; Smith, F.; Stacey, M.; Tatlow, J. C. (1952). "The High-Temperature Dimerisation of Chlorotrifluoroethylene". Journal of the Chemical Society (Resumed): 3830. doi:10.1039/JR9520003830.
- R. E. Putnam, B. C. Anderson, W. H. Sharkey (1963). "1-Chloro-1,4,4-Trifluorobutadiene". Organic Syntheses. 43: 17. doi:10.15227/orgsyn.043.0017.
{{cite journal}}: CS1 maint: multiple names: authors list (link)