Zeatin
Zeatin is a cytokinin derived from adenine, which occurs in the form of a cis- and a trans-isomer and conjugates. Zeatin was discovered in immature corn kernels from the genus Zea. It promotes growth of lateral buds and when sprayed on meristems stimulates cell division to produce bushier plants.
 | |
| Names | |
|---|---|
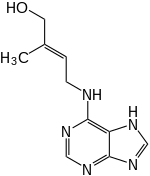
| IUPAC name
(E)-2-methyl-4-(7H-purin-6-ylamino)but-2-en-1-ol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C10H13N5O | |
| Molar mass | 219.248 g·mol−1 |
| Appearance | Off-white to yellow crystalline powder |
| Melting point | 208 to 210 °C (406 to 410 °F; 481 to 483 K) |
| Solubility in 1M NaOH | Soluble |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
2200 mg/kg (mouse, transperitoneal) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Occurrence
Zeatin and its derivatives occur in many plant extracts and are the active ingredient in coconut milk, which causes plant growth.[1]
6-(γ,γ-Dimethylallylamino)purine is a zeatin precursor.[2]
Application
Zeatin has a variety of effects including:
- Promotes callus initiation when combined with auxin, concentration 1 ppm.
- Promotes fruit set. Zeatin 100 ppm + GA3 500 ppm + NAA 20 ppm, sprayed at 10th, 25th, 40th day after blossom.
- Retards yellowing for vegetables, 20 ppm, sprayed.
- Causes auxiliary stems to grow and flower.
Zeatin can also be applied to stimulate seed germination and seedling growth.
Zeatin has also been shown to promote the resistance of tobacco against the bacterial pathogen Pseudomonas syringae, in which trans-zeatin has a more prominent effect than cis-zeatin. [3]
References
- David W. S. Mok; Machteld C. Mok (1994). Cytokinins: Chemistry, Activity, and Function. CRC Press. p. 8. ISBN 0-8493-6252-0.
- "6-(γ,γ-Dimethylallylamino)purine BioReagent, suitable for plant cell culture, 1 mg/mL". MilliporeSigma | United States. Retrieved 2021-10-12.
- Großkinsky, D.K.; Edelsbrunner, K.; Pfeifhofer, H.; van der Graaff, E. & Roitsch, T. (2013). "Cis- and trans-zeatin differentially modulate plant immunity". Plant Signaling & Behavior. 8 (7): e24798. doi:10.4161/psb.24798. PMC 3906432. PMID 23656869.