Citraconic acid
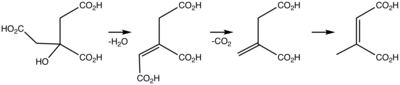
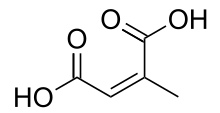
Citraconic acid is an organic compound with the formula CH3C2H(CO2H)2. It is a white solid. The alkene is cis. The related trans alkene is called mesaconic acid. It is one of the pyrocitric acids formed upon the heating of citric acid.[1] Citraconic acid can be produced, albeit inefficiently, by oxidation of xylene and methylbutanols. The acid displays the unusual property of spontaneously forming the anhydride, which, unlike maleic anhydride, is a liquid at room temperature.[2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2Z)-2-Methylbut-2-enedioic acid | |
| Other names
2-Methylmaleic acid Citraconate Methylmaleic acid cis-Methylbutenedioic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.145 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C5H6O4 | |
| Molar mass | 130.099 g·mol−1 |
| Appearance | Monoclinic crystals[1] |
| Density | 1.62 g/cm3[1] |
| Melting point | ~90 °C (decomposition)[1] |
| Freely soluble[1] | |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302 | |
| P264, P270, P301+P312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In the laboratory, citraconic acid can be produced by thermal isomerization of itaconic acid anhydride to give citraconic anhydride, which can be hydrolyzed to citraconic acid.[3] The required itaconic acid anhydride is obtained by dry distillation of citric acid.
References
- Budavari, Susan, ed. (1996), The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (12th ed.), Merck, ISBN 0911910123
- Kurt Lohbeck; Herbert Haferkorn; Werner Fuhrmann; Norbert Fedtke. "Maleic and Fumaric Acids". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_053.
- R. L. Shriner; S. G. Ford; l. J. Roll (1931). "Citraconic Anhydride and Citraconic Acid". Org. Synth. 28: 28. doi:10.15227/orgsyn.011.0028.