Clofibric acid
Clofibric acid is a biologically active metabolite of the lipid-lowering drugs clofibrate, etofibrate and theofibrate[1][2] with the molecular formula C10H11ClO3. It has been found in the environment following use of these drugs, for example in Swiss lakes and the North Sea.[2][3]
 | |
| Names | |
|---|---|
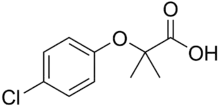
| Preferred IUPAC name
2-(4-Chlorophenoxy)-2-methylpropanoic acid | |
| Other names
Clofibrin Chlorofibrinic acid Chlorophenoxyisobutyric acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.751 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H11ClO3 | |
| Molar mass | 214.645 g/mol |
| Appearance | White to yellow solid |
| Melting point | 118 to 123 °C (244 to 253 °F; 391 to 396 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Some derivatives of clofibric acid are in a drug class called fibrates.
See also
- Phenoxy herbicides to which the compound is chemically related
References
- Salgado, R.; Oehmen, A.; Carvalho, G.; Noronha, J.P.; Reis, M.A.M. (2012). "Biodegradation of clofibric acid and identification of its metabolites". Journal of Hazardous Materials. 241–242: 182–189. doi:10.1016/j.jhazmat.2012.09.029. PMID 23062606.
- Packer, Jennifer L; Werner, Jeffrey J; Latch, Douglas E; McNeill, Kristopher; Arnold, William A (2003). "Photochemical fate of pharmaceuticals in the environment: Naproxen, diclofenac, clofibric acid, and ibuprofen". Aquatic Sciences. 65 (4): 342–351. doi:10.1007/s00027-003-0671-8. S2CID 24063392.
- Buser, Hans-Rudolf; Müller, Markus D; Theobald, Norbert (1998). "Occurrence of the Pharmaceutical Drug Clofibric Acid and the Herbicide Mecoprop in Various Swiss Lakes and in the North Sea". Environmental Science & Technology. 32 (1): 188–192. Bibcode:1998EnST...32..188B. doi:10.1021/es9705811.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.