Coprinellus micaceus
Coprinellus micaceus, commonly known as the mica cap, glistening inky cap, or shiny cap, is a common species of mushroom-forming fungus in the family Psathyrellaceae with a cosmopolitan distribution. The fruit bodies of the saprobe typically grow in clusters on or near rotting hardwood tree stumps or underground tree roots. Depending on their stage of development, the tawny-brown mushroom caps may range in shape from oval to bell-shaped to convex, and reach diameters up to 3 cm (1+1⁄4 in). The caps, marked with fine radial or linear grooves that extend nearly to the center, rest atop whitish stipes up to 10 cm (4 in) long. In young specimens, the entire cap surface is coated with a fine layer of reflective mica-like cells. Although small and with thin flesh, the mushrooms are usually bountiful, as they typically grow in dense clusters. A few hours after collection, the gills will begin to slowly dissolve into a black, inky, spore-laden liquid—an enzymatic process called autodigestion or deliquescence. The fruit bodies are edible before the gills blacken and dissolve, and cooking will stop the autodigestion process.
| Coprinellus micaceus | |
|---|---|
 | |
| Mushrooms near Erbach an der Donau, Germany | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Fungi |
| Division: | Basidiomycota |
| Class: | Agaricomycetes |
| Order: | Agaricales |
| Family: | Psathyrellaceae |
| Genus: | Coprinellus |
| Species: | C. micaceus |
| Binomial name | |
| Coprinellus micaceus | |
| Synonyms[1] | |
| |
| Coprinellus micaceus | |
|---|---|
| Gills on hymenium | |
| Cap is conical or campanulate | |
| Hymenium is adnexed | |
| Stipe is bare | |
| Spore print is black | |
| Ecology is saprotrophic | |
| Edibility is edible | |
The microscopic characteristics and cytogenetics of C. micaceus are well known, and it has been used frequently as a model organism to study cell division and meiosis in basidiomycetes. Chemical analysis of the fruit bodies has revealed the presence of antibacterial and enzyme-inhibiting compounds. Formerly known as Coprinus micaceus, the species was transferred to Coprinellus in 2001 as phylogenetic analyses provided the impetus for a reorganization of the many species formerly grouped together in the genus Coprinus. Based on external appearance, C. micaceus is virtually indistinguishable from C. truncorum, and it has been suggested that many reported collections of the former may be of the latter.
History and taxonomy

Coprinellus micaceus was illustrated in a woodcut by the 16th-century botanist Carolus Clusius in what is arguably the first published monograph on fungi, the 1601 Rariorum plantarum historia (History of rare plants), in an appendix,[2][3] Clusius erroneously believed the species to be poisonous, and classified it as a genus of Fungi perniciales (harmful fungi). The species was first described scientifically by French botanist Jean Baptiste François Pierre Bulliard in 1786 as Agaricus micaceus in his work Herbier de la France.[4] In 1801, Christian Hendrik Persoon grouped together all of the gilled fungi that auto-digested (deliquesced) during spore discharge into the section Coprinus of the genus Agaricus.[5] Elias Magnus Fries later raised Persoon's section Coprinus to genus rank in his Epicrisis Systematis Mycologici, and the species became known as Coprinus micaceus.[6] It was the type species of subsection Exannulati in section Micacei of the genus Coprinus, a grouping of related taxa with veils made of sphaerocysts (round swollen cells usually formed in clusters) exclusively or with thin-filamentous connective hyphae intermixed.[7] Molecular studies published in the 1990s[8][9] demonstrated that many of the coprinoid (Coprinus-like) mushrooms were in fact unrelated to each other. This culminated in a 2001 revision of the genus Coprinus, which was split into four genera; C. micaeus was transferred to Coprinellus.[10]
Due partly to their ready availability and the ease with which they may be grown in the laboratory, C. micaceus and other coprinoid mushrooms were common subjects in cytological studies of the 19th and 20th centuries. The German botanist Johann Heinrich Friedrich Link reported his observations of the structure of the hymenium (the fertile spore-bearing surface) in 1809,[11] but misinterpreted what he had seen. Link thought that microscopic structures known today as basidia were thecae, comparable in form to the asci of the Ascomycetes, and that each theca contained four series of spores. His inaccurate drawings of the hymenium of C. micaceus were copied in subsequent mycological publications by other authors, and it was not until microscopy had advanced that mycologists were able to determine the true nature of the basidia, when nearly three decades later in 1837 Joseph-Henri Léveillé and August Corda independently published correct descriptions of the structure of the hymenium.[3] In 1924, A. H. Reginald Buller published a comprehensive description and analysis of the processes of spore production and release in the third volume of his Researches on Fungi.[12]
The specific epithet micaceus is derived from the Latin word mica, for "crumb, grain of salt" and the suffix -aceus, "like, similar";[13] the modern application of "mica" to a very different substance comes from the influence of micare, "glitter".[14] The mushroom is commonly known as the "shiny cap",[15] the "mica cap" or the "glistening inky cap", all in reference to the mealy particles found on the cap that glisten like mica.[16]
Description


The cap is initially 1–2.5 cm (1⁄2–1 in) in diameter, oval to cylindrical, but expands to become campanulate (bell-shaped), sometimes with an umbo (a central nipple-like protrusion); finally it flattens somewhat, becoming convex. When expanded, the cap diameter reaches .8–5 cm (1⁄4–2 in) with the margin torn into rays and turned upwards slightly. The color is yellow-brown or tan often with a darker center, then pale yellow or buff from the margin inwards.[17] The cap margin is prominently grooved almost all the way to the center; the grooves mark the positions of the longer gills on the underside of the cap. When young, the cap surface is covered with white or whitish shiny particles, remnants of the universal veil that covers immature specimens.[18] The particles are loosely attached and easily washed away, so that older specimens are often smooth.[19] Coprinellus micaceus is hygrophanous, meaning it assumes different colors depending on its state of hydration.[20]
The gills are crowded together closely, and have an adnexed (narrow) attachment to the stipe.[21] Initially white, they change color to dark brown then eventually black as the spores mature. Expansion of the cap causes the gills to split open down their median planes, tearing the cap margin into rays. The process of spore discharge and autodigestion begin at the bottom of the gills before the upper parts of the gills have become completely blackened.[22] The brittle stipe is hollow, and measures 3–10 cm (1+1⁄4–4 in) long by 2–5 mm (1⁄16–3⁄16 in) thick and is roughly the same diameter throughout the length of the stipe. It is generally white but may discolor to pale dirty cream from the base up.[17] The stipe surface is at first velvety with a very fine whitish powder, but this eventually wears off, leaving it more or less smooth. Stipes may have a rudimentary ring at the base, another universal veil remnant.[16] The spore print is dark brown or black.[23] The flesh is thin, fragile, white in the stipe, and brownish in the cap.[24] Its odor and taste are not distinctive.[25] Individual fruit bodies take an average of five to seven days to fully mature.[26]
Microscopic characteristics
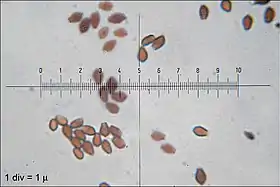
The spores of C. micaceus are reddish-brown or black,[17] with dimensions of 7–10 by 4.5–6 µm. Generally, they are lentiform (shaped like a biconvex lens), but viewed from the side they appear more almond-shaped or spindle-shaped, while in front view they appear oval or mitriform (roughly the shape of a miter—a peaked cap). Spores have a germ pore, a flattened area in the center of the spore surface through which a germ tube may emerge.[23] The spore-bearing cells (the basidia) are four-spored, club-shaped, and measure 10–15 by 4–7 µm.[27] Studies have shown that the basidia develop in four discrete generations. The first generation basidia are the most protuberant and extend out the greatest distance from the surface of the hymenium. Subsequent generations of basidia have shorter and less protuberant bodies. When a living gill is viewed with a microscope, the four sets of basidia can be seen distinctly. Arthur Buller coined the term inaequihymeniiferous to describe this mode of hymenial development. The purpose of the staggered basidia sizes is to facilitate the release of spores from the hymenium. There are four zones of spore discharge that correspond to the four sets of basidia, and basidia that have released all of their spores quickly begin to autodigest. The staggered setup minimizes the chance of spores colliding with neighboring basidia during release.[28]
Cystidia that are located along the edge of the cap (called cheilocystidia) are spherical, and 30–120 by 20–74 µm. The facial cystidia (called pleurocystidia) are club-shaped or elongated ellipses, up to 130–155 µm in length. The pleurocystidia protrude from the face of the gill and act as guards, preventing adjacent gills from touching each other, and also ensuring that the basidia and spores have sufficient room for development.[29] C. micaceus may also have scattered caulocystidia (cystidia on the stipe) that are 60–100 by 5–10 µm, but their presence is variable and cannot reliably be used for identification.[27] Both De Bary and Buller, in their investigations into the structure of the cystidia, concluded that there is a central mass of cytoplasm formed where numerous thin plates of cytoplasm meet at the center of the cell. De Bary believed that the plates were filamentous branching processes,[30] but Buller thought that they were formed in a process similar to the walls of foam bubbles and that the central mass was able to slowly change form and position by altering the relative volumes of the vacuoles enclosed by the numerous thin cytoplasmic walls. In older cells, the cytoplasm may be limited to the periphery of the cell, with one huge vacuole occupying the cell center.[31]
The globular cells that make up the mica-resembling scales on the cap are colorless, smooth-walled, and range in size from about 25–65 µm, although most are between 40 and 50 µm.[22] Buller explained the "glitter" of these cells as follows: "The sparkling of the meal-cells, as well as of the cystidia on the edges and faces of the gills, is simply due to light which strikes them from without and is refracted and reflected to the eye in the same manner as from the minute drops of water one so often sees at the tips of grass leaves on English lawns early in the morning after a dewy night."[32]
In 1914, Michael Levine was the first to report successfully cultivating C. micaceus from spores in the laboratory. In his experiments, fruit bodies appeared roughly 40 to 60 days after initially inoculating the growth media (agar supplemented with soil, horse dung, or cornmeal) with spores.[33] Like other coprinoid species, C. micaceus undergoes synchronous meiosis. The chromosomes are readily discernible with light microscopy, and all of the meiotic stages are well-defined. These features have made the species a useful tool in laboratory investigations of Basidiomycete cytogenetics.[34][35] The chromosome number of C. micaceus is n=12.[36]
Edibility
Coprinellus micaceus is an edible species,[21][37] and cooking inactivates the enzymes that cause autodigestion or deliquescence—a process that can begin as soon as one hour after collection.[38] It is considered good for omelettes,[24] and as a flavor for sauces,[15] although it is "a very delicate species easily spoiled by overcooking".[39] The flavor is so delicate that it is easy to overpower and hide with almost anything. The fungus also appeals to fruit flies of the genus Drosophila, who frequently use the fruit bodies as hosts for larvae production.[40][41]
A study of the mineral contents of various edible mushrooms found that C. micaceus contained the highest concentration of potassium in the 34 species tested, close to half a gram of potassium per kilogram of mushroom.[42] Because the species can bioaccumulate detrimental heavy metals like lead and cadmium, it has been advised to restrict consumption of specimens collected from roadsides or other collection sites that may be exposed to or contain pollutants.[43]
Similar species

The edible Coprinellus bisporus is nearly identical but lacks the yellowish cap granules and only has two spores per basidium. The scaly inky cap (Coprinus variegatus = Coprinus quadrifidus) has a grayish-brown cap with dull white to brownish scales; its odor is disagreeable. The trooping crumble cap (Coprinellus disseminatus, edible) has smaller, yellow-brown to grey-brown caps and white gills that turn black but do not dissolve away; it always grows in large clusters on rotting wood (sometimes buried wood).[44] Coprinopsis atramentaria is a larger,[45] gray species that grows in dense clusters on stumps or on the ground from buried wood, lacks glistening particles on the cap, and the cap and gills dissolve at maturity. Coprinellus radians develops singly or in clumps on wood, from a tufted mat of coarse yellow-orange mycelium.[46] Coprinellus truncorum is also covered with glistening granules and is said to be almost indistinguishable from C. micaceus in the field; microscopy is needed to tell the difference, as C. truncorum has ellipsoid spores with a rounded germ pore, compared to the shield-shaped (mitriform) spores with truncated germ pores of C. micaceus.[47] One study suggests that compared to C. truncorum, C. micaceus is browner in the center of the cap (rather than grayish) and has a greater tendency to grow in clusters; more molecular evidence is required to determine if the two taxa are genetically identical.[27] C. flocculosus is another similar species.[17]
Ecology, habitat and distribution
Coprinellus micaceus is a saprotrophic species, deriving nutrients from dead and decomposing organic matter, and grows in and around stumps or logs of broad-leaved trees or attached to buried wood. It prefers feeding on bark, particularly the secondary phloem, rather than the wood.[48] In the scheme of the succession of fungal species involved in the decomposition of wood, C. micaceus is a late stage colonizer, and prefers to feed on wood that has already decomposed sufficiently to have reached "a friable softened consistency".[49] A 2010 study suggests that the fungus can also live as an endophyte, inhabiting the woody tissue of healthy trees without causing disease symptoms.[50] The fungus is also associated with disturbed or developed ground, such as the sides of roads and paths, gardens, building sites and the edges of parking lots;[51] it has also been noted for growing indoors on rotting wood in humid environments.[16] In one instance it was discovered about 120 m (400 ft) underground in an abandoned coal mine, growing on wooden gangways and props used to support the roof.[52]

Fruit bodies are commonly found growing in dense clusters, but can also be found growing singly or in small clumps, especially in forested areas.[20] In North America, C. micaceus is one of the first edible mushrooms to appear in the spring,[25] and fruits from April to September. In Europe, it fruits from May to December.[39] Although it can grow at any time of the year, it is more prevalent during the spring and fall, coinciding with the higher humidity resulting from spring and autumn rains.[46] A study of air quality conducted in the city of Santiago de Compostela in the Iberian Peninsula, concluded that most "Coprinus" spores present in the atmosphere belonged to C. micaceus, and that the number of spores went up with increased humidity and rainfall, but decreased with greater temperatures.[53] The species is known for reappearing with successive fruitings at the same location. In one case, a total of 38 lb (17.2 kg) of fresh mushrooms were collected from one elm stump in 10 successive crops over a spring and summer.[54][55]
Coprinellus micaceus has a cosmopolitan distribution,[24] and has been collected in northern Africa,[56] South Africa,[57] Europe (including Turkey[58]), North America (as far north as Alaska),[59] the Hawaiian islands,[27][47] South America,[27] India,[60][61] Australia,[39] New Zealand,[62] and Japan.[63] Phylogenetic analysis of rDNA sequences from specimens collected in southeastern Asia and Hawaii show that the Hawaiian species form a distinct clade with little genetic diversity compared to Asian populations; this suggests that the Hawaiian populations have been introduced relatively recently and have not had much time to develop genetic variation.[64] One study suggests that in South Africa, where C. micaceus is rare, it has been frequently confused with the similar-appearing C. truncorum, a more common species in that region. A similar inference has been raised about North American species.[59][65]
Bioactive compounds
Research into the natural product chemistry of Coprinellus micaceus has revealed the presence of several chemical compounds unique to the species. Micaceol is a sterol with "modest" antibacterial activity against the pathogens Corynebacterium xerosis and Staphylococcus aureus. The compound (Z,Z)-4-oxo-2,5-heptadienedioic acid has inhibitory activity against glutathione S-transferase, an enzyme that has been implicated in the resistance of cancer cells against chemotherapeutic agents, especially alkylating drugs.[66][67] A 2003 study did not find any antibacterial activity in this species.[68] A 1962 publication reported the presence of the biologically active indole compound tryptamine in C. micaceus, although the concentration was not determined.[69] The fruit bodies additionally produce a variety of pigment compounds known as melanins—complex chemical polymers that contribute to the formation of soil humus after the fruit bodies have disintegrated.[70] C. micaceus has been found to be devoid of the toxin coprine, the disulfiram-mimicking chemical found in Coprinopsis atramentaria that causes illness when consumed simultaneously with alcohol.[71]
References
- "Species synonymy – Coprinellus micaceus". Index Fungorum. CAB International. 2008. Archived from the original on 2011-06-10. Retrieved 2010-04-14.
- Fungorum in Pannoniis observatorum brevis historia (Brief history of fungi observed in Pannonia [Hungary]), genus XVI, p. cclxxxii.
- Buller, 1924, p. 331.
- Bulliard JBF (1786). Herbier de la France [Guide to the Herbs of France] (in French). Vol. 6. pp. 241–88, plate 246. Click on page 11.
- Persoon CH (1801). Synopsis Methodica Fungorum (Göttingen) (in Latin). Vol. 1. p. 403.
- Fries EM (1838). Epicrisis Systematis Mycologici (in Latin). Uppsala, Sweden: W.G. Farlow. p. 247.
- Singer R. (1986). The Agaricales in Modern Taxonomy (4th ed.). Königstein im Taunus, Germany: Koeltz Scientific Books. p. 522. ISBN 3-87429-254-1.
- Hopple JS, Vilgalys R (1994). "Phylogenetic relationships among coprinoid taxa and allies based on data from restriction site mapping of nuclear rDNA" (PDF). Mycologia. 86 (1): 96–107. doi:10.2307/3760723. JSTOR 3760723.
- Park DS, Go SJ, Kim YS, Seok SJ, Song JK, Yeo YS, Ryu JC, Sung JM (1999). "Genetic relationships of Coprinus spp. on the basis of sequences in ITS II region". Korean Journal of Mycology (in Korean). 27 (1): 27–31. ISSN 0253-651X.
- Redhead SA, Vilgalys R, Moncalvo J-M, Johnson J, Hopple JS Jr (2001). "Coprinus Pers. and the disposition of Coprinus species sensu lato". Taxon. 50 (1): 203–41. doi:10.2307/1224525. JSTOR 1224525.
- Link HF (1809). "Nova plantarum genera e classe Lichenum, Algarum, Fungorum". Schrader's Neues Journal für die Botanik. 3: 10–15.
- Buller, 1924, pp. 328–56.
- Stearn WT (1973). Botanical Latin (2nd annot. and rev. ed.). Newton Abbot: David & Charles. pp. 379, 463.
- Oxford English Dictionary Online. Oxford: Oxford University Press. 2010. ss. vv. "mica", "micaceous".
- Ammirati JF, McKenny M, Stuntz DE (1987). The New Savory Wild Mushroom. Seattle, Washington: University of Washington Press. p. 156. ISBN 0-295-96480-4.
- Arora, 1986, p. 348 Retrieved 2010-04-16.
- Davis, R. Michael; Sommer, Robert; Menge, John A. (2012). Field Guide to Mushrooms of Western North America. Berkeley: University of California Press. pp. 205–206. ISBN 978-0-520-95360-4. OCLC 797915861.
- Evenson VS (1997). Mushrooms of Colorado and the Southern Rocky Mountains. Westcliffe Publishers. p. 131. ISBN 978-1-56579-192-3.
- Buller, 1924, p. 335.
- McKnight VB, McKnight KH (1987). A Field Guide to Mushrooms, North America. Boston: Houghton Mifflin. p. 34. ISBN 0-395-91090-0.
- Orr DB, Orr RT (1979). Mushrooms of Western North America. Berkeley, California: University of California Press. p. 176. ISBN 0-520-03656-5.
- Buller, 1924, p. 337.
- Orton PD, Watling R (1979). Coprinaceae: Coprinus. Edinburgh, Scotland: Royal Botanic Garden. pp. 54–55. ISBN 0-11-491565-2.
- Schalkwijk-Barendsen HME. (1991). Mushrooms of Western Canada. Edmonton, Canada: Lone Pine Publishing. p. 334. ISBN 0-919433-47-2.
- Roody WC (2003). Mushrooms of West Virginia and the Central Appalachians. Lexington, Kentucky: University Press of Kentucky. p. 156. ISBN 0-8131-9039-8.
- Pady SM (1941). "Notes on Coprinus micaceus growing in an unusual habitat". Mycologia. 33 (4): 411–14. doi:10.2307/3754895. JSTOR 3754895.
- Keirle MR, Hemmes DE, Desjardin DE (2004). "Agaricales of the Hawaiian Islands. 8. Agaricaceae: Coprinus and Podaxis; Psathyrellaceae: Coprinopsis, Coprinellus and Parasola" (PDF). Fungal Diversity. 15 (4): 33–124.
- Buller, 1924, pp. 351–53.
- Buller, 1924, pp. 329, 342.
- De Bary A. (1873). "On cystidia". Grevillea. 1: 181–83.
- Buller, 1924, pp. 248–50.
- Buller, 1924, p. 340.
- Levine M. (1914). "The origin and development of the lamellae of Coprinus micaceus". American Journal of Botany. 1 (7): 343–56. doi:10.2307/2435139. JSTOR 2435139.
- Bos CJ (1996). Fungal Genetics: Principles and Practice. New York: Marcel Dekker. pp. 152–53. ISBN 0-8247-9544-X.
- Moore D, Chiu S-W (1996). Patterns in Fungal Development. Cambridge, UK: Cambridge University Press. p. 127. ISBN 0-521-56047-0.
- Lu BC, Raju NB (1970). "Meiosis in Coprinus. II. Chromosome pairing and the lampbrush diplotene stage of meiotic prophase". Chromosoma (Berlin). 29 (3): 305–16. doi:10.1007/BF00325945. PMID 5445348. S2CID 12287280.
- Russell B. (2006). Field Guide to Wild Mushrooms of Pennsylvania and the Mid-Atlantic. University Park, Pennsylvania: Pennsylvania State University Press. p. 29. ISBN 978-0-271-02891-0.
- Smith AH (1975). A Field Guide to Western Mushrooms. Ann Arbor, Michigan: University of Michigan Press. p. 229. ISBN 0-472-85599-9.
- Dickinson C, Lucas J (1982). VNR Color Dictionary of Mushrooms. New York: Van Nostrand Reinhold. p. 78. ISBN 978-0-442-21998-7.
- Kimura MT (1980). "Evolution of food preferences in fungus-feeding Drosophila: an ecological study". Evolution. 34 (5): 1009–18. doi:10.2307/2408009. JSTOR 2408009. PMID 28581130.
- Toda MJ, Kimura MT, Tuno N (1999). "Coexistence mechanisms of mycophagous drosophilids on multispecies fungal hosts: aggregation and resource partitioning". Journal of Animal Ecology. 68 (4): 794–803. doi:10.1046/j.1365-2656.1999.00328.x. JSTOR 2647329.
- Dursun N, Ozcan MM, Ozturk C (2006). "Mineral contents of 34 species of edible mushrooms growing wild in Turkey". Journal of the Science of Food and Agriculture. 86 (7): 1087–94. Bibcode:2006JSFA...86.1087D. doi:10.1002/jsfa.2462.
- Das N. (2007). "Heavy metal levels in wild edible mushroom samples from Nayagram Block of Midnapore District, West Bengal". Indian Forester. 133 (2): 171–78.
- Sundberg W, Bessette A (1987). Mushrooms: A Quick Reference Guide to Mushrooms of North America (Macmillan Field Guides). New York: Collier Books. p. 134. ISBN 0-02-063690-3.
- Trudell, Steve; Ammirati, Joe (2009). Mushrooms of the Pacific Northwest. Timber Press Field Guides. Portland, OR: Timber Press. p. 196. ISBN 978-0-88192-935-5.
- Healy RA, Huffman DR, Tiffany LH, Knaphaus G (2008). Mushrooms and Other Fungi of the Midcontinental United States (Bur Oak Guide). Iowa City, Iowa: University of Iowa Press. p. 63. ISBN 978-1-58729-627-7.
- Hemmes DE, Desjardin D (2002). Mushrooms of Hawai'i: An Identification Guide. Berkeley, California: Ten Speed Press. p. 127. ISBN 1-58008-339-0.
- Buller, 1924, p. 333.
- Peiris D, Dunn WB, Brown M, Kell DB, Roy I, Hedger JN (2008). "Metabolite profiles of interacting mycelial fronts differ for pairings of the wood decay basidiomycete fungus, Stereum hirsutum with its competitors Coprinus micaceus and Coprinus disseminatus". Metabolomics. 4 (1): 52–62. doi:10.1007/s11306-007-0100-4. S2CID 22052503.
- de Errasti A, Carmarán CC, Novas MV (2010). "Diversity and significance of fungal endophytes from living stems of naturalized trees from Argentina". Fungal Diversity. 40 (1): 29–40. doi:10.1007/s13225-009-0012-x. S2CID 30981477.
- Arora, 1986, p. 48. Retrieved 2010-04-13.
- Atkinson GF (1898). "Society for Plant Morphology and Physiology. 2. Studies on some mycelium and fungi from a coal mine". Botanical Gazette. 25 (2): 106–18. doi:10.1086/327641. JSTOR 2464466. S2CID 224843835.
- Aira MJ, Rodriguez-Rajo FJ, Castro M, Jato V (2009). "Characterization of Coprinus spores in the NW of the Iberian Peninsula. Identification and count in aerobiological samples". Cryptogamie, Mycologie. 30 (1): 57–66.
- Christensen CM (1981). Edible Mushrooms. Minnesota, Minneapolis: University of Minnesota Press. p. 80. ISBN 978-0-8166-1049-5.
- Stewart FC (1926). "The mica ink-cap or glistening Coprinus". New York (Geneva) Agricultural Experiment Station Bulletin. 535: 1–30.
- Melchers LE (1931). "A check list of plant diseases and fungi occurring in Egypt". Transactions of the Kansas Academy of Science. 34: 41–106. doi:10.2307/3624470. JSTOR 3624470.
- Reid DA, Eicker A (1999). "South African fungi 10: New species, new records and some new observations". Mycotaxon. 73: 169–97.
- Gezer K, Ekici FT, Turkoglu A (2008). "Macrofungi of Karci Mountain (Denizli, Turkey)". Turkish Journal of Botany. 32 (1): 91–6. ISSN 1300-008X.
- Watling R, Miller OK (1971). "8 species of Coprinus of Yukon Territory and adjacent Alaska". Canadian Journal of Botany. 49 (9): 1687–90. doi:10.1139/b71-237.
- Kaul TN, Kachroo JL (1974). "Common edible mushrooms of Jammu and Kashmir India". Journal of the Bombay Natural History Society. 71 (1): 26–31.
- Manjula R, Surjit S, Dutta BB, Krishnendu A (2005). "Some additions to the Coprinaceae of Sikkim Himalaya". Journal of Mycopathological Research. 43 (1): 101–03. ISSN 0971-3719.
- Crowe A. (1983). A Field Guide to the Native Edible Plants of New Zealand : including Those Plants Eaten by the Maori. Collins. p. 135. ISBN 0-00-216983-5.
- Uljé, K. "Coprinus micaceus". Kees Uljé Coprinus site. Retrieved 2010-04-14.
- Ko KS. (2001). "Phylogeographic divergences of nuclear ITS sequences in Coprinus species sensu lato". Mycological Research. 105 (12): 1519–26. doi:10.1017/S0953756201005184.
- Kuo M. (February 2008). "Coprinellus micaceus". MushroomExpert.Com. Retrieved 2011-03-11.
- Zahid S, Udenigwe CC, Ata A, Eze MO, Segstro EP, Holloway P (2006). "New bioactive natural products from Coprinus micaceus". Natural Product Research. 20 (14): 1283–89. doi:10.1080/14786410601101829. PMID 17393652. S2CID 7494031.
- Ata A. (2009). "Novel bioactive natural products from marine and terrestrial sources". In Şener B (ed.). Innovations in Chemical Biology. Dordrecht, The Netherlands: Springer Netherlands. pp. 51–60. doi:10.1007/978-1-4020-6955-0. ISBN 978-1-4020-6954-3.
- Efremenkova OV, Ershova EY, Tolstych IV, Zenkova VA, Dudnik YV (2003). "Antimicrobial activity of medicinal mushrooms from the genus Coprinus (Fr.) S. F. Gray (Agaricomycetideae)". International Journal of Medicinal Mushrooms. 5 (1): 37–41. doi:10.1615/InterJMedicMush.v5.i1.
- Worthen LR, Stessel GJ, Youngken HW (1962). "The occurrence of indole compounds in Coprinus species". Economic Botany. 16 (4): 315–18. doi:10.1007/BF02860190. S2CID 22240178.
- Saiz-Jimenez C. (1983). "The chemical nature of the melanins from Coprinus spp". Soil Science. 136 (2): 65–74. Bibcode:1983SoilS.136...65S. doi:10.1097/00010694-198308000-00001. hdl:10261/76183. S2CID 26206774.
- Brossi A. (1991). The Alkaloids: Chemistry and Pharmacology. Vol. 40. Amsterdam, the Netherlands: Elsevier. p. 298. ISBN 0-12-469540-X.
Cited books
- Arora D. (1986). Mushrooms Demystified: a Comprehensive Guide to the Fleshy Fungi. Berkeley, California: Ten Speed Press. ISBN 0-89815-169-4.
- Buller AH (1924). "Chapter XI: The micaceus sub-type illustrated by Coprinus micaceus". Researches on Fungi. Vol. III. London: Longmans, Green, and Co. pp. 328–56.
External links
 Media related to Coprinellus micaceus at Wikimedia Commons
Media related to Coprinellus micaceus at Wikimedia Commons Data related to Coprinellus micaceus at Wikispecies
Data related to Coprinellus micaceus at Wikispecies