Criegee intermediate
A Criegee intermediate (also called a Criegee zwitterion or Criegee biradical) is a carbonyl oxide with two charge centers. These chemicals may react with sulfur dioxide and nitrogen oxides in the earth's atmosphere, and are implicated in the formation of aerosols, which are an important factor in controlling global climate.[1][2] Criegee intermediates are also an important source of OH (hydroxyl radicals).[3] OH radicals are the most important oxidant in the troposphere,[4] and are important in controlling air quality and pollution.
.svg.png.webp)
The formation of this sort of structure was first postulated in the 1950s by Rudolf Criegee,[5] for whom it is named. It was not until 2012 that direct detection of such chemicals was reported.[6] Infrared spectroscopy suggests the electronic structure has a substantially zwitterionic character rather than the biradical character that had previously been proposed.[7]
Formation
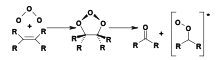
Criegee intermediates are formed by the gas-phase reactions of alkenes and ozone in the earth's atmosphere. Ozone adds across the carbon–carbon double bond of the alkene to form a molozonide, which then decomposes to produce a carbonyl (RR'CO) and a carbonyl oxide. The latter is known as the Criegee intermediate.[8]
The alkene ozonolysis reaction is extremely exothermic, releasing about 50 kilocalories per mole (210 kJ/mol) of excess energy. Therefore, the Criegee intermediates are formed with a large amount of internal energy.[8]
Removal
When Criegee intermediates are formed, some portion of them will undergo prompt unimolecular decay, producing OH radicals and other products. However, they may instead become stabilized by interactions with other molecules or react with other chemicals to give different products.
Criegee intermediates may be collisionally stabilized via collisions with other molecules in the atmosphere. These stabilized Criegee intermediates may then undergo thermal unimolecular decay to OH radicals and other products, or may undergo bimolecular reactions with other atmospheric species.
In the ozonolysis reaction sequence, the Criegee intermediate reacts with another carbonyl compound (generally the aldehyde or ketone byproduct of the Criegee-intermediate formation reaction itself) to form an ozonide (1,2,4-trioxolane).
References
- Welz, Oliver; Savee, John D.; Osborn, David L.; Vasu, Subith S.; Percival, Carl J.; Shallcross, Dudley E.; Taatjes, Craig A. (13 January 2012). "Direct Kinetic Measurements of Criegee Intermediate (CH2OO) Formed by Reaction of C2I with O2". Science. 335 (6065): 204–207. Bibcode:2012Sci...335..204W. doi:10.1126/science.1213229. PMID 22246773. S2CID 26810853.
- Castro, Joseph (January 12, 2012). "How mysterious molecules may help cool Earth". NBC News. Retrieved 2012-01-12.
- Heard, Dwayne E.; Whalley, Lisa K.; Stone, Daniel (2012). "Tropospheric OH and HO2 radicals: field measurements and model comparisons". Chemical Society Reviews. 41 (19): 6348–6404. doi:10.1039/C2CS35140D. PMID 22907645.
- Finlayson-Pitts, Barbara J.; Pitts, James N. (2000). Chemistry of the upper and lower atmosphere : theory, experiments, and applications. San Diego: Academic Press. ISBN 9780080529073. OCLC 162128929.
- "Offsetting Global Warming: Molecule in Earth's Atmosphere Could 'Cool the Planet'". Science Daily. January 12, 2012. Retrieved 2012-01-14.
- Taatjes, Craig A.; Shallcross, Dudley E.; Percival, Carl J.; Vasu, Subith S.; Osborn, David L.; Savee, John D.; Welz, Oliver (2012-01-13). "Direct Kinetic Measurements of Criegee Intermediate (CH2OO) Formed by Reaction of CH2I with O2". Science. 335 (6065): 204–207. Bibcode:2012Sci...335..204W. doi:10.1126/science.1213229. ISSN 1095-9203. PMID 22246773. S2CID 26810853.
- Su, Yu-Te; Huang, Yu-Hsuan; Witek, Henryk A.; Lee, Yuan-Pern (12 April 2013). "Infrared Absorption Spectrum of the Simplest Criegee Intermediate CH2OO". Science. 340 (6129): 174–176. Bibcode:2013Sci...340..174S. doi:10.1126/science.1234369. PMID 23580523. S2CID 31499893.
- Marston, George; Johnson, David (2008-03-25). "The gas-phase ozonolysis of unsaturated volatile organic compounds in the troposphere". Chemical Society Reviews. 37 (4): 699–716. doi:10.1039/B704260B. ISSN 1460-4744. PMID 18362978.