Cyclochlorotine
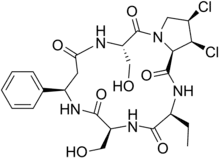
Cyclochlorotine[1] (CC), also known as islanditoxin[2] is a mycotoxin produced by the fungus Penicillium islandicum[3] that causes liver damage and has carcinogenic properties.[4] Japanese researchers confirmed that it was one of three strains of Penicillin fungi responsible for yellowed rice.[2] It is listed as an IARC Group 3 carcinogen.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,2-Dichloro-15-ethyl-5,12-bis-hydroxymethyl-9-phenyl-dodecahydro-3a,6,10,13,16-pentaaza-cyclopentac
yclohexadecene-4,7,11,14,17-pentaone | |
| Other names
Cyclo[(R)-3-phenyl-β-alanyl-L-seryl-(2α,3α,4α)-3,4-dichloro-L-prolyl-L-2-aminobutanoyl-L-seryl]; Yellowed rice toxin | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C24H31Cl2N5O7 | |
| Molar mass | 572.44 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Chemically, it is a dichlorinated cyclic peptide.[5] Structurally, the only thing that differentiates cyclochlorotine from the plant-derived astins of Aster tataricus, is replacement of a serine with a second 2-aminobutyrate.[6]
Cyclochlorotine are one of the toxins usually found in foods in the grains group like rice, wheat, soybeans, peanuts, beans, bread, flour, etc. Such foods serve as medium for the growth of molds like Penicillium islandicum which in turn releases toxins like cyclochlorotine etc.[7] Research shows the detailed biosynthesis of Cyclochlorotine which is a multi step mechanism and makes use of a vital component in the last step known as NRPS ( CctN) which helps produce the desired Cyclochlorotine. [6]
References
- Zhou, ZH; Komiyama, M; Terao, K; Shimada, Y (1994). "Effects of cyclochlorotine on myofibrils in cardiomyocytes and on actin filament bundles in fibroblasts in vitro". Nat. Toxins. 2 (6): 378–85. doi:10.1002/nt.2620020607. PMID 7704452.
- Kushiro, Masayo (2015). "Historical review of researches on yellow rice and mycotoxigenic fungi adherent to rice in Japan". JSM Mycotoxins. 65: 12–23. doi:10.2520/myco.65.19.
- "Toxicology of Penicillium islandicum". Nature. 191 (4791): 864–865. 1961. Bibcode:1961Natur.191..864.. doi:10.1038/191864b0. S2CID 38045877.
- Penicillium islandicum causes hepatic necrosis and has carcinogenic properties
- Kohei Mizutani; Yusuke Hirasawa; Yoshiko Sugita-Konishi; Naoki Mochizuki; Hiroshi Morita (2008). "Structural and conformational analysis of hydroxycyclochlorotine and cyclochlorotine, chlorinated cyclic peptides from Penicillium islandicum". J. Nat. Prod. 71 (7): 1297–1300. doi:10.1021/np800150m. PMID 18558744.
- Schafhauser, Thomas; Kirchner, Norbert; Kulik, Andreas; Huijbers, Mieke M. E.; Flor, Liane; Caradec, Thibault; Fewer, David P.; Gross, Harald; Jacques, Philippe (2016-11-01). "The cyclochlorotine mycotoxin is produced by the nonribosomal peptide synthetase CctN in Talaromyces islandicus (Penicillium islandicum)" (PDF). Environmental Microbiology. 18 (11): 3728–3741. doi:10.1111/1462-2920.13294. ISSN 1462-2920. PMID 26954535. S2CID 22896792.
- Gosh, Anil; Manmade, Awinash; Townsend, James; Bousquet, Ann; Howes, John; Demain, Arnold (June 1978). "Production of Cyclochlorotine and a New Metabolite, Simatoxin, by Penicillium islandicum Sopp". American Society for Microbiology. 35 (6): 1074–1078. doi:10.1128/AEM.35.6.1074-1078.1978. PMC 242987. PMID 677874.