Cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occurs as a colorless liquid with a petrol-like odor. Its freezing point is −94 °C and its boiling point is 49 °C. Cyclopentane is in the class of cycloalkanes, being alkanes that have one or more carbon rings. It is formed by cracking cyclohexane in the presence of alumina at a high temperature and pressure.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclopentane | |||
| Other names
pentamethylene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.470 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C5H10 | |||
| Molar mass | 70.1 g/mol | ||
| Appearance | clear, colorless liquid | ||
| Odor | mild, sweet | ||
| Density | 0.751 g/cm3 | ||
| Melting point | −93.9 °C (−137.0 °F; 179.2 K) | ||
| Boiling point | 49.2 °C (120.6 °F; 322.3 K) | ||
| 156 mg·l−1 (25 °C)[1] | |||
| Solubility | soluble in ethanol, acetone, ether | ||
| Vapor pressure | 45 kPa (20 °C) [2] | ||
| Acidity (pKa) | ~45 | ||
| -59.18·10−6 cm3/mol | |||
Refractive index (nD) |
1.4065 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Flammable[3] | ||
| NFPA 704 (fire diamond) | |||
| Flash point | −37.2 °C (−35.0 °F; 236.0 K) | ||
| 361 °C (682 °F; 634 K) | |||
| Explosive limits | 1.1%-8.7%[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[3] | ||
REL (Recommended) |
TWA 600 ppm (1720 mg/m3)[3] | ||
IDLH (Immediate danger) |
N.D.[3] | ||
| Related compounds | |||
Related compounds |
cyclopropane, cyclobutane, cyclohexane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
It was first prepared in 1893 by the German chemist Johannes Wislicenus.[4]
Production, occurrence and use
Cycloalkanes are formed by catalytic reforming. For example, when passed over a hot platinum surface, 2-methylbutane converts into cyclopentane.
Cyclopentane has found applications in various industries. As a volatile hydrocarbon it is an incidental component of some fuels and blowing agents. In recent years, cyclopentane has been used as a refrigerant to replace chlorofluorocarbons (CFCs) and hydrofluorocarbons (HFCs) as it does not deplete the ozone layer and has a much lower global warming potential than HFC refrigerants. Cyclopentane requires safety precautions to prevent leakage and ignition when used as a refrigerant as it is highly flammable.
Cyclopentane can be fluorinated to give compounds ranging from C5H9F to perfluorocyclopentane C5F10. Such species are conceivable refrigerants and specialty solvents.[5][6]
The cyclopentane ring is pervasive in natural products including many useful drugs. Examples include most steroids, prostaglandins, and some lipids.
Conformations
In a regular pentagon the angles at the vertices are all 108°, slightly less than the bond angle in perfectly tetrahedrally bonded carbon, which is about 109.47°. But cyclopentane is not planar in its normal conformations. It puckers in order to increase the distances between the hydrogen atoms (something which does not happen in the planar cyclopentadienyl anion C5H−5 because it doesn't have as many hydrogen atoms). This means that the average C-C-C angle is less than 108°. There are two conformations that give local minima of the energy, the "envelope" and the "half-chair". The envelope has mirror symmetry (Cs), while the half chair has two-fold rotational symmetry (C2). In both cases the symmetry implies that there are two pairs of equal C-C-C angles and one C-C-C angle that has no pair. In fact for cyclopentane, unlike for cyclohexane (C6H12, see cyclohexane conformation) and higher cycloalkanes, it is not possible geometrically for all the angles and bond lengths to be equal except if it is in the form of a flat regular pentagon.
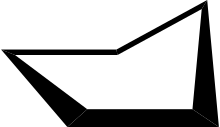 Envelope
Envelope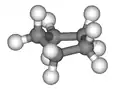 3D envelope
3D envelope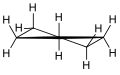 Half-chair
Half-chair
References
- Record of cyclopentane in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 28 February 2015.
- "ICSC 0353 - CYCLOPENTANE".
- NIOSH Pocket Guide to Chemical Hazards. "#0171". National Institute for Occupational Safety and Health (NIOSH).
- J. Wislicenus and W. Hentschel (1893) "Der Pentamethenylalkohol und seine Derivate" (Cyclopentanol and its derivatives), Annalen der Chemie, 275 : 322-330; see especially pages 327-330. Wislicenus prepared cyclopentane from cyclopentanone ("Ketopentamethen"), which is prepared by heating calcium adipate.
- Tatlow, John Colin (1995). "Cyclic and bicyclic polyfluoro-alkanes and -alkenes". Journal of Fluorine Chemistry. 75 (1): 7–34. doi:10.1016/0022-1139(95)03293-m. ISSN 0022-1139.
- Zhang, Chengping; Qing, Feiyao; Quan, Hengdao; Sekiya, Akira (January 2016). "Synthesis of 1,1,2,2,3,3,4-heptafluorocyclopentane as a new generation of green solvent". Journal of Fluorine Chemistry. 181: 11–16. doi:10.1016/j.jfluchem.2015.10.012.
External links
 Media related to Cyclopentane at Wikimedia Commons
Media related to Cyclopentane at Wikimedia Commons