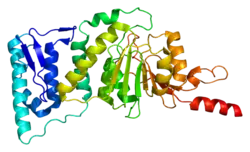
DOT1L
DOT1-like (Disruptor of telomeric silencing 1-like), histone H3K79 methyltransferase (S. cerevisiae), also known as DOT1L, is a protein found in humans, as well as other eukaryotes.[5][6] The methylation of histone H3 lysine 79 (H3K79) by DOT1L which is a conserved epigenetic mark in many eukaryotic epigenomes, increases progressively along the aging process, suggesting that "DOT1L might function as a vital clock, ticking the hours impassively".[7]
DOT1L has been reported to play an important role in the processes of mixed-lineage leukemia (MLL)-rearranged leukemias[8]
Small molecule inhibitors of Dot1L catalytic activity have been developed.[9][10]
All three forms of H3K79 methylation (H3K79me1; H3K79me2; H3K79me3) are catalyzed by DOT1 in yeast or DOT1L in mammals. H3K79 methylation participates in the DNA damage response and has multiple roles in nucleotide excision repair and sister chromatid recombinational repair.[11]
References
- GRCh38: Ensembl release 89: ENSG00000104885 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000061589 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: DOT1L DOT1-like, histone H3 methyltransferase (S. cerevisiae)".
- Vlaming H, van Leeuwen F (2016). "The upstreams and downstreams of H3K79 methylation by DOT1L". Chromosoma. 125 (4): 1–13. doi:10.1007/s00412-015-0570-5. PMID 26728620. S2CID 18876283.
- Soria-Valles C, Osorio FG, López-Otín C (2015). "Reprogramming aging through DOT1L inhibition". Cell Cycle. 14 (21): 3345–3346. doi:10.1080/15384101.2015.1093443. PMC 4825545. PMID 26375309.
- Slany RK (2016). "The molecular mechanics of mixed lineage leukemia". Oncogene. 35 (40): 5215–5223. doi:10.1038/onc.2016.30. PMID 26923329. S2CID 34116587.
- Yao Y, Chen P, Diao J, Cheng G, Deng L, Anglin JL, Prasad BV, Song Y (2011). "Selective inhibitors of histone methyltransferase DOT1L: design, synthesis, and crystallographic studies". Journal of the American Chemical Society. 133 (42): 16746–9. doi:10.1021/ja206312b. PMC 3492951. PMID 21936531.
- Chen S, Li L, Chen Y, Hu J, Liu J, Liu YC, Liu R, Zhang Y, Meng F, Zhu K, Lu J, Zheng M, Chen K, Zhang J, Jiang H, Yao Z, Luo C (2016). "Identification of Novel Disruptor of Telomeric Silencing 1-like (DOT1L) Inhibitors through Structure-Based Virtual Screening and Biological Assays". Journal of Chemical Information and Modeling. 56 (3): 527–34. doi:10.1021/acs.jcim.5b00738. PMID 26914852.
- Chen Y, Zhu WG (July 2016). "Biological function and regulation of histone and non-histone lysine methylation in response to DNA damage". Acta Biochim. Biophys. Sin. (Shanghai). 48 (7): 603–16. doi:10.1093/abbs/gmw050. PMID 27217472.
Further reading
- Nagase T, Nakayama M, Nakajima D, Kikuno R, Ohara O (April 2001). "Prediction of the coding sequences of unidentified human genes. XX. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro". DNA Research. 8 (2): 85–95. doi:10.1093/dnares/8.2.85. PMID 11347906.
- Feng Q, Wang H, Ng HH, Erdjument-Bromage H, Tempst P, Struhl K, Zhang Y (June 2002). "Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain". Current Biology. 12 (12): 1052–8. doi:10.1016/S0960-9822(02)00901-6. PMID 12123582. S2CID 17263035.
- Min J, Feng Q, Li Z, Zhang Y, Xu RM (March 2003). "Structure of the catalytic domain of human DOT1L, a non-SET domain nucleosomal histone methyltransferase". Cell. 112 (5): 711–23. doi:10.1016/S0092-8674(03)00114-4. PMID 12628190. S2CID 17822742.
- Jikuya H, Takano J, Kikuno R, Hirosawa M, Nagase T, Nomura N, Ohara O (February 2003). "Characterization of long cDNA clones from human adult spleen. II. The complete sequences of 81 cDNA clones". DNA Research. 10 (1): 49–57. doi:10.1093/dnares/10.1.49. PMID 12693554.
- Okada Y, Feng Q, Lin Y, Jiang Q, Li Y, Coffield VM, Su L, Xu G, Zhang Y (April 2005). "hDOT1L links histone methylation to leukemogenesis". Cell. 121 (2): 167–78. doi:10.1016/j.cell.2005.02.020. PMID 15851025. S2CID 15638573.
- Sistayanarain A, Tsuneyama K, Zheng H, Takahashi H, Nomoto K, Cheng C, Murai Y, Tanaka A, Takano Y (2006). "Expression of Aurora-B kinase and phosphorylated histone H3 in hepatocellular carcinoma". Anticancer Research. 26 (5A): 3585–93. PMID 17094487.
External links
- Overview of all the structural information available in the PDB for UniProt: Q8TEK3 (Histone-lysine N-methyltransferase, H3 lysine-79 specific) at the PDBe-KB.